Calculating the Required Mass of a Reactant
Q1.How much zinc carbonate would we need to decompose to produce 24.2 g of zinc oxide.
Q2. 28.5 g of calcium carbonate reacts with an excess of sulphuric acid to form calcium sulphate, carbon dioxide and water. Calculate the (theoretical) mass of calcium sulphate that will be formed in this reaction.
Q3. Calculate the mass of potassium hydroxide that would be needed to form 50 g of potassium nitrate in the neutralisation reaction between nitric acid and potassium hydroxide.
Q4. The alcohol ethanol can be produced from the alkene gas ethene (ethylene) during the hydrolysis reaction as shown:
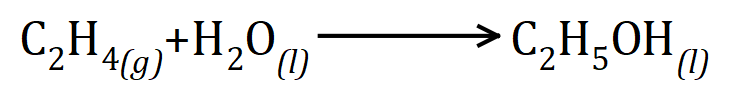
Calculate the mass of ethene that would be needed to make 240 g of ethanol using this reaction.
Q5. One of the methods to produce iron from dioxide is by reduction with carbon, producing iron metal from the oxide and carbon dioxide. The equation for the reaction is as shown will stop given the equation calculate the amount of iron oxide which would be needed to produce 96 g of iron metal:
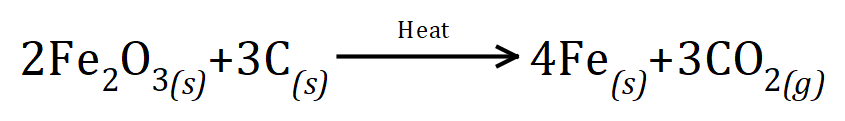
Go To >> Solutions <<
Back To >> Calculating the Required Mass of a Reactant <<