Calculating the Required Mass of a Reactant
Q1.How much zinc carbonate would we need to decompose to produce 24.2 g of zinc oxide.
The answer to this question will be the theoretical maximum as aforementioned, but let us take our first step which should always be the balanced chemical equation:

We are told in the question that we are interested in the mass of zinc oxide, as the carbon dioxide is effectively being disregarded we will perform no mathematics on it. the relative atomic mass of zinc is 65, carbon is 12 and oxygen is 16. Therefore the relative molecular mass of zinc carbonate is 125. By a similar calculation the relative molecular mass of zinc oxide is 65+16 = 81.
From this point it is a matter of proportions, we can see that one mole of zinc carbonate will decompose to produce 1 mole of zinc oxide and 1 mole of carbon dioxide. If we started with 125 g of zinc carbonate we would theoretically end with 81 g of zinc oxide and 44 g of carbon dioxide. If we add 81 and 44 together we get back to 125 which once again proves the law of conservation of mass is obeyed. We are told in the question that we are required to produce 24.2 g of zinc oxide which is 24.2÷81 or approximately 0.29 moles (I said approximately because the answer was 0.298 continuing infinitely with decimal places. You will usually be told how many decimal places to work to).
To produce 0.29 moles of zinc oxide at a theoretical 100% yield we must start off with the same number of moles of zinc carbonate. 0.29 moles is 0.29×125= 36.25 g and this is the answer to the question.
Q2. 28.5 g of calcium carbonate reacts with an excess of sulphuric acid to form calcium sulphate, carbon dioxide and water. Calculate the (theoretical) mass of calcium sulphate that will be formed in this reaction.
Once again, I cannot stress this enough, start with the balanced chemical reaction:

You can see that this is quite a straightforward equation, one mole of calcium carbonate will react with one mole of sulphuric acid to produce one mole of calcium sulphate one mole of carbon dioxide and one mole of water. We now need to establish the relative molecular masses of calcium carbonate and calcium sulphate as these are the only 2 substances that the question is interested in. The relative molecular mass of calcium carbonate is 40+12+ (3x16)= 100 and the relative molecular mass of calcium sulphate is 40+32+ (4×16)=136.
We can see therefore that if we were working in molar quantities, 100 g of calcium carbonate would ultimately produce 136 g of calcium sulphate, but we are in fact working with 28.5 g of calcium carbonate which is 28.5÷100= 0.285 moles. From the one-to-one relationship of the equation we can see therefore that theoretically we should produce 0.285 moles of calcium sulphate, which is 0.285×136=38.8g.
Q3. Calculate the mass of potassium hydroxide that would be needed to form 50 g of potassium nitrate in the neutralisation reaction between nitric acid and potassium hydroxide.
This question gives you the bare bones, the expectation is that you are no or can work out the missing information for you to complete the calculation. Once again, always start with the balanced chemical reaction:

This is quite a simple neutralisation, one mole of nitric acid in aqueous solution will neutralise one mole of potassium hydroxide (this could either be solid dropped into the acid, or in aqueous solution) to produce one mole of potassium nitrate in aqueous solution and a mole of water. Our requirements are to produce 50 g of potassium nitrate. the relative atomic masses of potassium, oxygen and hydrogen are 39, 16 and 1 respectively and the relative atomic mass of nitrogen is 14. This will give you enough information to carry out the rest of the calculation viz:
One mole of potassium hydroxide would have a mass of 39+16+1= 56 g and this would produce 39+14+(3x16) = 101 g. We are asked how much potassium hydroxide to use to produce 50 g or 50÷101= 0.495 moles. Using the relative molecular mass of potassium hydroxide as 56, we can establish that we would in fact need to use 0.495×56= 27.72 g of potassium hydroxide.
Q4. The alcohol ethanol can be produced from the alkene gas ethene (ethylene) during the hydrolysis reaction as shown:
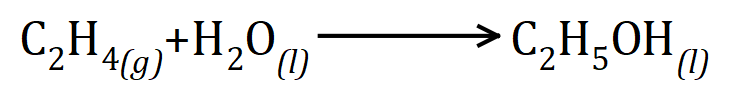
Calculate the mass of ethene that would be needed to make 240 g of ethanol using this reaction.
This time you are given the balanced chemical reaction which once again is quite straightforward, one mole of ethene will react with one mole of water (steam) to produce 1 mole of ethanol. the relative atomic masses of carbon hydrogen and oxygen are 12, 1 and 16 respectively. The relative molecular mass of ethene is therefore 28, the relative molecular mass of water is 18 and the relative molecular mass of ethanol is 46. We can therefore state that 28 g of ethene would react with 18 g of water to produce 46 g of ethanol. We are being asked to produce 240 g of ethanol which is 240÷46 = 5.22 moles. We therefore require 5.22 moles of ethene which is 5.22×28=146g
Q5. One of the methods to produce iron from dioxide is by reduction with carbon, producing iron metal from the oxide and carbon dioxide. The equation for the reaction is as shown will stop given the equation calculate the amount of iron oxide which would be needed to produce 96 g of iron metal:
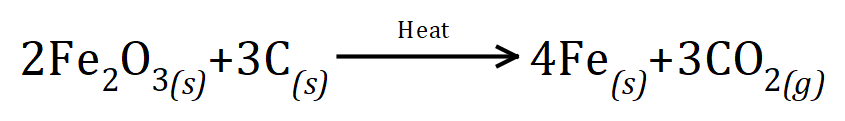
In keeping with all of the questions so far, from the equation we can see that 2 moles of iron oxide will react with 3 moles of carbon to produce 4 moles of iron and 3 moles of carbon dioxide gas. We are not given the relative atomic masses so this is one of those questions where you will be required to find these yourself, you will usually be given a periodic table and/or an equation sheet in your test/examination. For the purposes of this question the relative atomic mass of iron is 56, the relative atomic mass of oxygen is 16 and the relative atomic mass of carbon is 12. We now have all the information we need to attack the question.
Using molar quantities we can see that 2 moles of iron oxide (which is 2 times the relative molecular mass of iron oxide, or 2 x (56+56+16+16+16) = 320g would react with 36 g of carbon to produce 4×56 = 224g iron and 3×44 = 132 g carbon dioxide. the question asks us how much iron oxide would be required to produce 96 g of iron and we can do this by a simple arithmetical calculation:
The amount of iron oxide required would be (96 / 224) x 320 = 137.1 g
An alternative way of calculating this would be to take note of the fact that to produce 4 moles of iron requires 2 moles of iron oxide, and we do in fact produce 96 g of iron which is 96÷56= 1.714 moles, with therefore require half of this number of moles in iron oxide which is 0.857 moles. Taking the relative molecular mass of iron oxide (160) and multiplying this by 0.857 leads to a result of 160×0.857= 137.14, rounding to the nearest single decimal point gives us 137.1 which is the answer obtained in the other method.
It should be quite obvious to you by now that these calculations involve relatively simple arithmetic, but the trick in arriving at the simple arithmetic is the seemingly complicated manipulation that you have to undergo to start with. Mastering this skill will bring in lots of points during tests and examinations!
Back To >> Questions <<
Back To >> Calculating the Required Mass of a Reactant <<