Limiting Reactants
Q1. When 4.48 g of iron was reacted with an excess of copper sulphate solution, 5.08 g of copper was produced. How much copper would be produced if we increased the amount of iron to 13.44 g but kept the copper sulphate in excess?
We are given the masses of iron as 4.48 increasing to 13.44, a factor of 3 but the copper sulphate remains in excess. If we therefore treble the amount of iron must also travel amount of copper produced so 5.08 g of copper would increase to 15.24 g.
It is also possible to calculate the mass of product expected in the reaction if we know the identity of the limiting reactant and the balanced symbolic equation for the reaction.
Q2. What would be the mass of aluminium oxide (alumina) produced if 135 g of aluminium was burned in air?
You should be able to identify the fact that the limiting reactant is of course aluminium, because you've been given a specific mass of it. "Burned in air" is a dead giveaway that oxygen will be in excess. Let us take a look at the balanced chemical reaction:
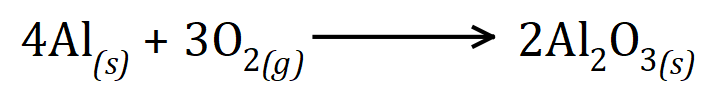
4 moles of aluminium will react with 3 moles of diatomic oxygen to produce 2 moles of aluminium oxide. We are told that we have 135 g of aluminium which is 135÷27 = 5 moles so using this information and the balanced chemical reaction you should be able to see that 2.5 moles of aluminium oxide would be produced as a reaction is a 4:2 ratio. using the relative molecular mass of aluminium oxide as 102 will give us 2.5×102 = 255 g
Calculations of this type are "easy mark makers" in an examination provided that you can get your head around the concept of moles and molar quantities. The next batch of questions will take you through this:
Q3. 3.25 g of zinc reacts with an excess of hydrochloric acid to produce 6.80 g of zinc chloride. what would happen if the amount of zinc was doubled but the hydrochloric acid remained in excess?
This is the sort of question you should be able to answer immediately, if the amount of zinc doubles therefore so should the amount of product in which case we will produce 13.6 g of zinc chloride if we double the amount of zinc.
Q4. Using the same scenario as above, how much zinc chloride will be produced if we dissolve exactly 13 g of zinc in excess hydrochloric acid?
This is where a good understanding of moles, molar quantities and relative atomic and formula/molecular masses comes in useful. the relative atomic mass of zinc is 65.4 and we have 13 g of it, so therefore we have 13÷65.4= 0.2 moles to 2 decimal places. looking at the balanced chemical equation for the reaction:
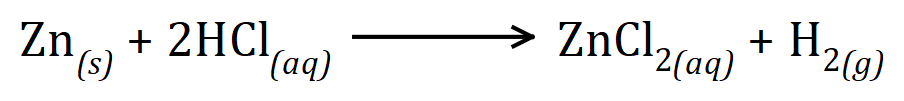
We can see purely from the zinc parts of the equation that one mole of zinc will lead to the production of one mole of zinc chloride, therefore 0.2 moles of zinc will logically lead to the production of 0.2 moles of zinc chloride. The relative molecular mass zinc chloride is 65.4+35.5+35.5 = 136.4 and so the amount produced in grams would be 0.2×136.4 = 27.28 g
Back To >> Questions <<
Back To >> Limiting Reactants <<