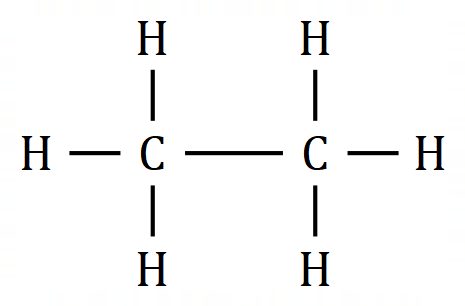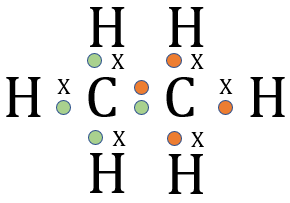[A] Naming Conventions (Nomenclature)
The naming conventions of organic compounds, or nomenclature, can become quite complicated. When we look at the simpler organic compounds such as the Alkanes, the smaller or "shorter chain" Alkanes are quite straightforward, however when we start to look at double bonded and triple bonded Alkenes and Alkynes and also where there are mixtures of "side chains" and double/triple bonds things can start to get messy.
|
|
|
|
|
3D Spatial Representation |
Wedge and Dash |
Displayed Formula |
|
CH3CH3 or C2H6 |
− |
|
|
Molecular |
Skeletal |
Dot and Cross Notation |
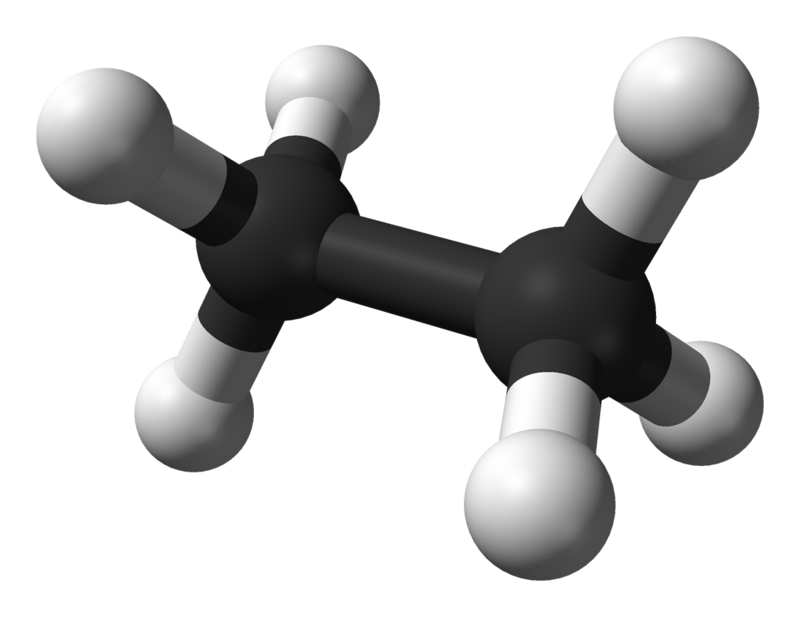
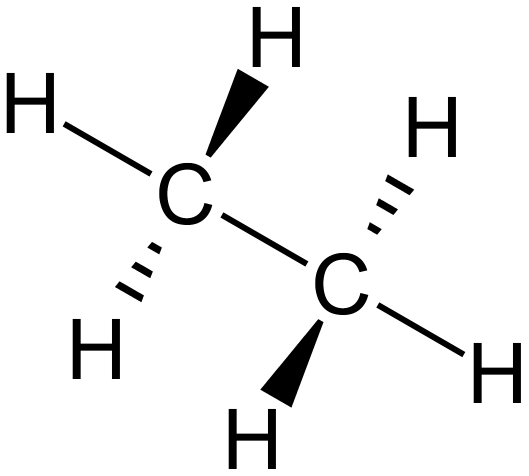
The above table of structural representations has been replicated from another section. When we are talking about small molecules it is sometimes interesting to use the "3D Spatial Representation" because although this method isn't strictly speaking very accurate (for various reasons) it does give an indication of the stereochemistry of the molecule. The "Wedge and Dash" representation simplifies the drawing of the molecule whilst trying to preserve a little bit of the stereochemistry. The "Displayed Formula" is okay for small to medium-size molecules, but when we start to look at large molecules the only real option in the interests of speed is the "Skeletal" representation. Now in the diagram above, what looks like a single "overgrown hyphen" is all that is used to depict a molecule of Ethane, however this "Skeletal" or "Line" representation starts to become a little bit more useful as the molecules get larger. Before we actually go into naming compounds, let's take a look at a larger molecule using the skeletal representation:
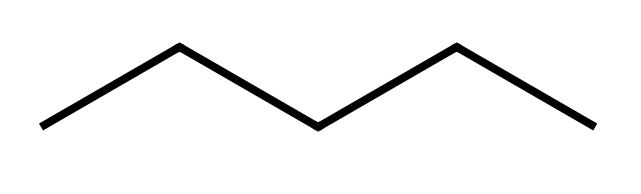
The "zigzag" structure is something that is necessary to show, because it's emphasises the bonding type between carbon atoms. Although it is out of the scope of this level of study, carbon atoms have some of their orbitals "blended together" to form new types of "hybrid" bonds, without taking this any further it is sufficient to say that these new "hybridised" bonds have a tetrahedral structure which gives rise to the zigzagging that you see.
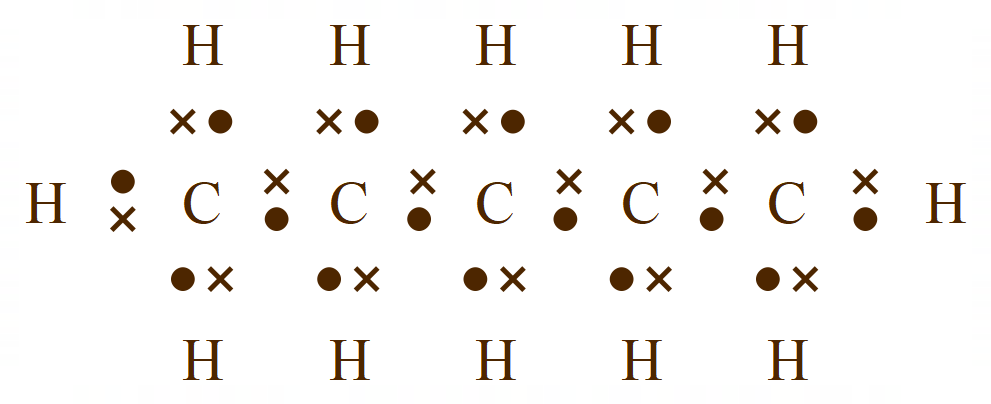
Normally, to sketch a molecule of pentane would take quite some time, we have to remember that as well as five carbon atoms there are twelve hydrogens to add, and these have to be shown in the correct numbers, in other words three each end of the chain and two at each of the carbon atoms in the middle. This is of course to satisfy the (four) valency of carbon, which is shown quite comfortably in the dot and cross representation but comes with the drawback of taking quite some time to sketch out.
>> Organic Nomenclature - Substituent Hierarchy <<