[A] Arenes
The intentions of the chemistry side of WEISAM is to try to stick as much as possible to the GCSE level syllabus, or its future equivalent. When we move into what is presently known as "year twelve" and "year thirteen" which are the A-level years, we start to look at more complicated chemical structures in organic chemistry. Here, I will briefly introduce a family of compounds which are the first circular organic molecules that you will come across. They are circular, but we prefer to use the term "cyclic", the first of which is Benzene.
In your studies of Alkenes, you will have no doubt come across substances called "dienes" and possibly even "trienes" which are hydrocarbon substances with two or three double bonds respectively.
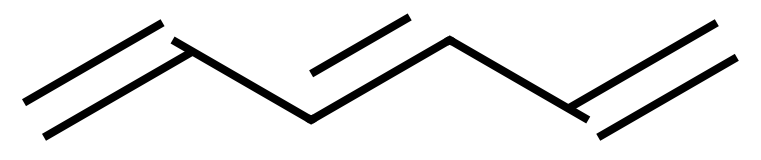
You can undoubtedly, by now, name this for yourself, but above shows a molecule of 1,3,5-hexatriene (Hexa-1,3,5-triene). If we were to "wrap this round into a circle" we would arrive at a very common depiction of the structure of Benzene:
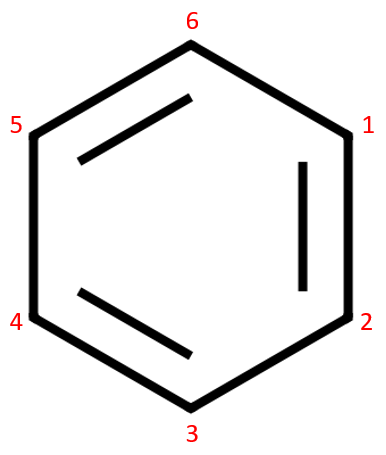
It's not as simple as this, the molecule above takes on the structure of a substance known as 1,3,5-cyclohexatriene which suggests that the double bonds as shown do in fact alternate between every other carbon. Some chemistry texts state that 1,3,5-cyclohexatriene is in fact Benzene, but that Benzene is not 1,3,5-cyclohexatriene!
Full explanations of this is way beyond the scope and intention of this document, but suffice it to say that studies have suggested that the alternating one, two, one, two, one, two bond representations in the above molecule are more likely to be "one and a half" all around the ring. This would give us a pictorial representation something like this:
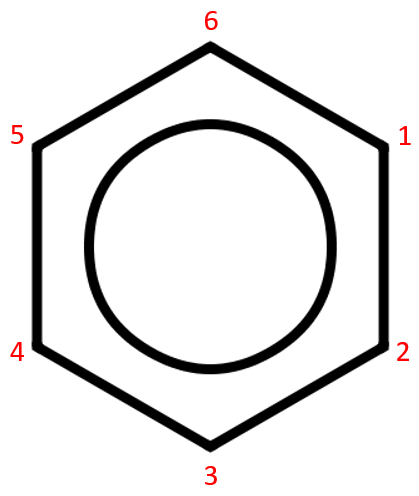
The "circle inside the hexagon" representation of benzene is intended to show the "resonance" of the single and double bonds, it might be easier to think of these as constantly alternating between one and two, averaging out at any time to "one and a half" bonds between carbon atoms.
I don't intend to go into any further detail as to the proposed bonding in the benzene ring as this would require an explanation of new types of bond (hybrid bonds) between carbon atoms which you have not yet come across. Probably best not to muddy the waters too much at this point.
Let's take a look at a "ball and stick" Benzene molecule:
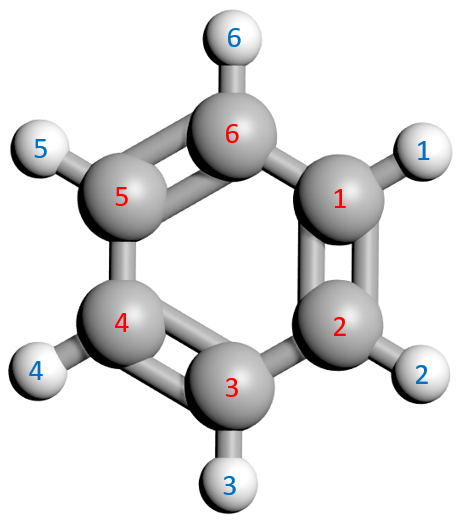
Each of the carbon atoms has been randomly numbered in red, I've not started at any particular point. Each corresponding hydrogen has been numbered in blue, again the fact that the numbers match is a few convenience for what I'm going to try to explain in the next paragraphs.
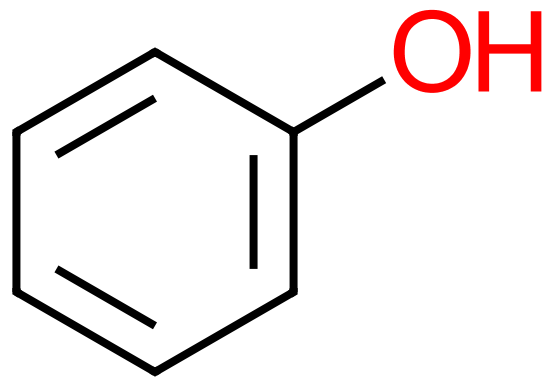
If we were to replace one of the hydrogen atoms with a hydroxyl group, we would have the first cyclic (aromatic) alcohol known as Phenol. Sometimes referred to as Hydroxybenzene, Phenol is a corrosive and poisonous substance, a white crystalline solid (but I have seen it turn pink due to oxidation) with a very strong and distinctive odour.
|
|
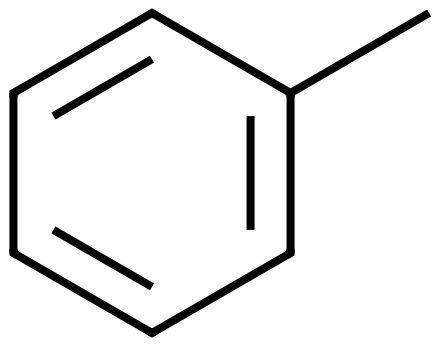
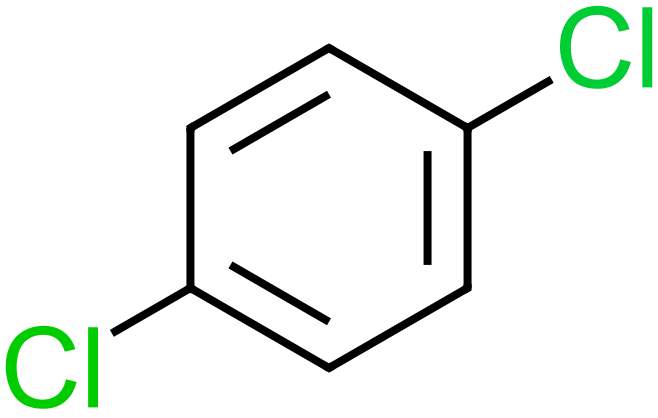
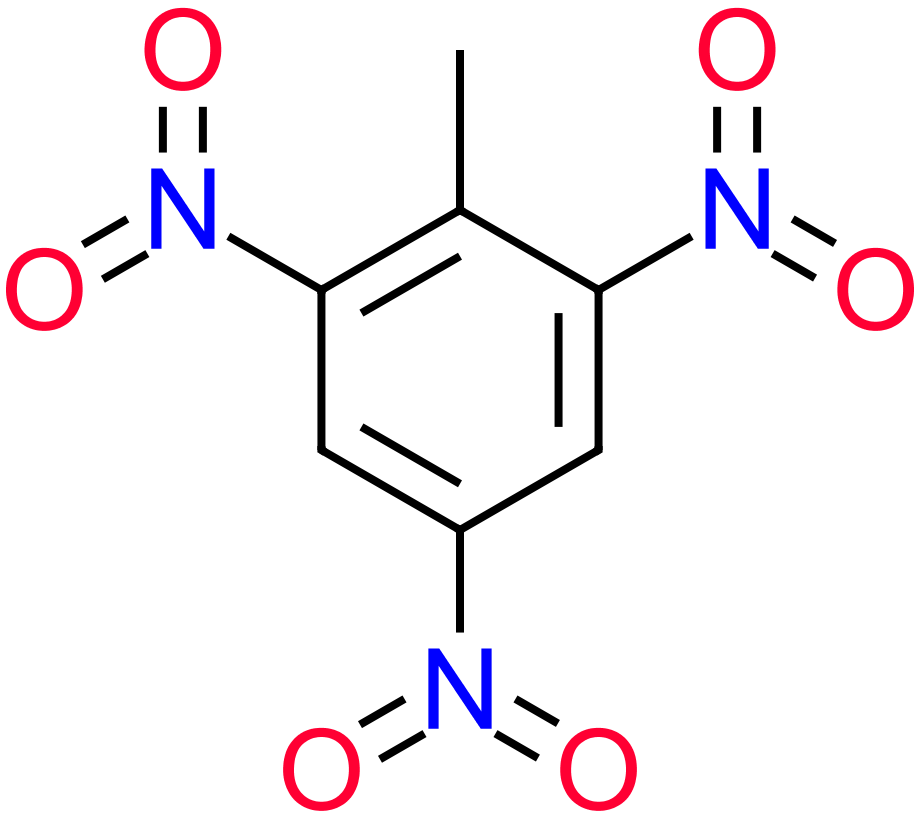
The four molecules on the left show some of the simpler, and not so simpler "substituted" Benzenes. Hydroxybenzene, otherwise known as Phenol is used as an antiseptic, in low doses as too much phenol is poisonous. It is used in substances such as "chloraseptic" which (at the time of writing anyway) is a fast acting local anaesthetic for extremely sore throats. Methylbenzene, otherwise known as toluene used as a general organic solvent, it needs to be treated with care as it is a colorless liquid with a sweet, pungent odor. Exposure to toluene can cause eye and nose irritation, tiredness, confusion, euphoria, dizziness, headache, dilated pupils, tears, anxiety, muscle fatigue, insomnia, nerve damage, inflammation of the skin, and liver and kidney damage. 1,4-Dichlorobenzene or "para" dichlorobenzene is a not unpleasant smelling disinfectant substance, used widely in sanitation (it is, or used to be, an active ingredient in the yellow disinfectant blocks found in urinals. The expression "para" relates to the relative positions of the two chlorine atoms. Two substituents on the benzene ring which are "side-by-side" in positions 1 and 2 would be regarded as "ortho", if they were "one carbon atom apart" and so therefore in positions 1 and 3 they would be "meta" and being diametrically opposite in the 1,4 position gives the prefix of "para". The chemistry behind the location of substituents with relation to each other on a benzene ring, and the conditions which influence such positions is beyond the scope of the intentions of this section. Finally, a slightly more complicated molecule which must indeed be handled with care if you're ever going to make it (highly unlikely in any academic setting I would have thought). 2,4,6-Trinitrotoluene, better known as TNT is a highly explosive substance. TNT is one of the most commonly used explosives for military, industrial, and mining applications. TNT has been used in conjunction with hydraulic fracturing (fracking), a process used to recover oil and gas from shale formations. The technique involves displacing and detonating nitroglycerin in hydraulically induced fractures followed by wellbore shots using pelletized TNT. |
|
Hydroxybenzene (Phenol) |
|
|
|
|
|
Methylbenzene (Toluene) |
|
|
|
|
|
1,4-Dichlorobenzene |
|
|
|
|
|
2,4,6-Trinitrotoluene |
|
|
|
TNT is valued partly because of its insensitivity to shock and friction, with reduced risk of accidental detonation compared to more sensitive explosives such as nitroglycerin. TNT melts at 80 °C (176 °F), far below the temperature at which it will spontaneously detonate, allowing it to be poured or safely combined with other explosives. TNT neither absorbs nor dissolves in water, which allows it to be used effectively in wet environments. To detonate, TNT must be triggered by a pressure wave from a starter explosive, called an explosive booster.