Alcohols
As you're probably aware by now, organic chemistry is full of "families" of compounds, the "functional group" being in the identifier as to which family they belong to. We saw previously the carboxylic acids have the COOH functional group, alcohols contain one or more OH (hydroxyl) functional groups.
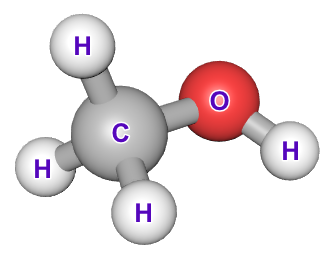
The molecule above, is the simplest of all of the alcohols, Methanol. A homologous series is formed when we consider the next in the series, Ethanol, then Propanol, Butanol and so forth.
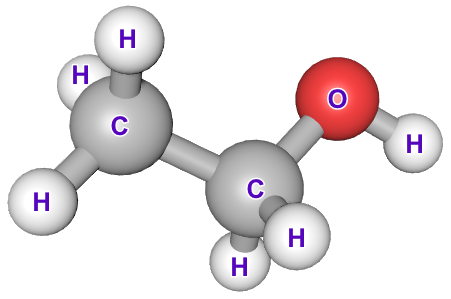
At the moment, it is fairly straightforward, Methanol is CH3OH and Ethanol is CH3CH2OH. In the straight chain alcohols, where the OH functional group lies at the end, the naming is quite straightforward based on the alkyl group, however when the alkyl groups start to get a bit longer there is the opportunity for them to branch, this can make the naming of alcohols (and indeed any organic compounds) a little bit more complicated because certain functional groups "outrank" others when it comes to the naming conventions (See the appendix Appendix C9 - Organic Nomenclature Substituent Group Hierarchy).
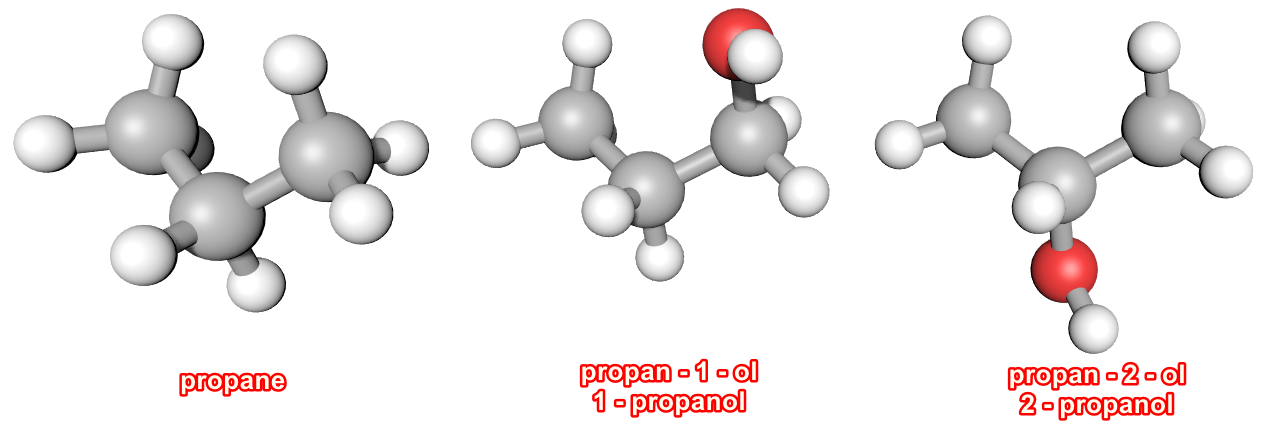
In the case of Methanol, the hydroxyl group can only go in one place, and in the case of Ethanol, although there are two places that the hydroxyl group can go to we end up with the same molecule (just turn it round). When we come to Propanol, we have two distinctly different environments to consider when we place the hydroxyl group. If we number the carbon atoms 1,2 and 3 we can see that numbers 1 and 3 will produce the same alcohol because it doesn't matter which end of the molecule the hydroxyl group is attached to, the result is still the same (as in the case of Ethanol). The alternative placement is to put the hydroxyl group on to carbon atom number 2.
We will have two distinct alcohols with the same molecular composition but a different structural arrangement, in other words we have isomers:
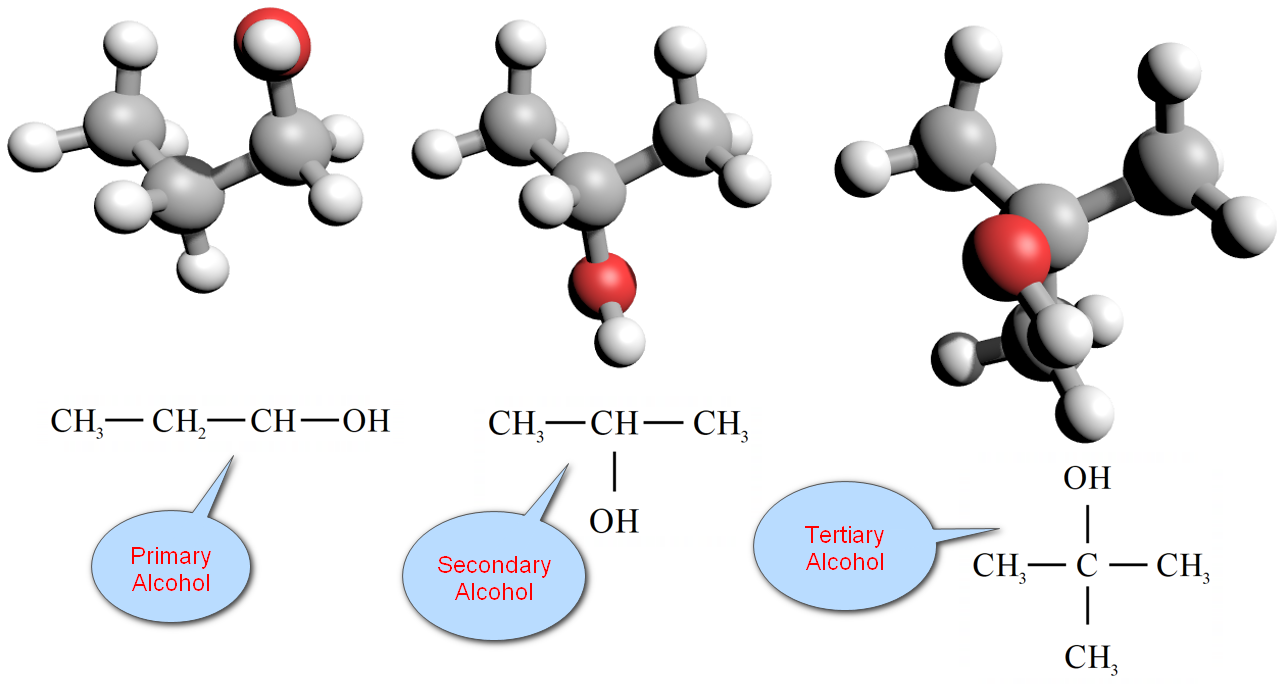
Primary alcohols
A primary alcohol is one in which the hydroxyl group (–OH) is attached to a carbon atom with at least two hydrogen atoms. This will only occur when the hydroxyl group is at the end of the molecule chain. Propan-1-ol is a primary alcohol.
Secondary alcohols
A secondary alcohol is one in which the hydroxyl group (-OH) is attached to a carbon with only one hydrogen atom attached. This can happen somewhere in the middle of a carbon chain. Propan-2-ol is a secondary alcohol.
Tertiary alcohols
A tertiary alcohol is one in which the hydroxyl group is attached to a carbon with no hydrogen atoms attached. This will normally mean that the hydroxyl group is joined to the same carbon atom as a branch. 2-methylpropan-2-ol is a tertiary alcohol.
Multiple OH groups