Chemical Equations
We have already talked about chemicals/elements forming mixtures and compounds. This particular area concerning chemical equations will show us how elements combine chemically to form compounds and in what proportions.
During chemical reactions bonds form or bonds break between atoms. Essentially what is happening is when compounds react together bonds between the atoms break and the atoms effectively change places. There are two ways in which we can show this process, by simple written "word" equations and by "symbolic" equations. The symbolic method is slightly more complicated because we have to make sure that we show the correct numbers of each atom involved in the reaction, whereas in the word equation all we need to do is show which elements react with which and what the resultant products are.
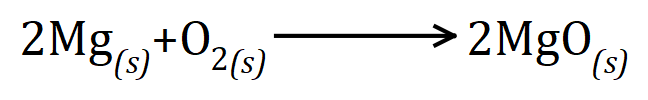
If we burn magnesium in oxygen, it burns with a brilliant blinding silvery white flame to form magnesium oxide:
The word equation is simply "magnesium plus oxygen gives magnesium oxide" and the symbolic version is shown below. Symbolically it is slightly more complicated because we need to show the molar quantities involved, in other words two moles of magnesium will react with one mole of oxygen molecules to produce two moles of magnesium oxide.
Q. Write out the word equation for the reaction between sodium and chlorine, producing sodium chloride.
A. Sodium plus chlorine gives sodium chloride
In written form, you can of course replace plus with its mathematical symbol, and the word "gives" can be replaced with a single headed arrow moving from left to right. You will need to do this by default when dealing with symbolic equations.
Balancing equations:
There must always be the same number of each type of atom on both sides of the chemical equation, this leads to us being able to say that the equation is "balanced". For example let us take a look once again at the reaction between sodium and chlorine which produces sodium chloride:
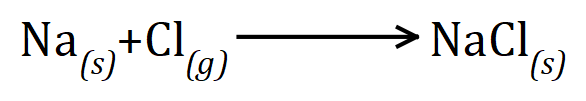
This appears to balance, because we have one atom of sodium on the left and one on the right, similarly we have one atom of chlorine on the left and one on the right. The reality is somewhat different in this particular case because chlorine is not a monatomic gas (chlorine atoms do not go around individually, they always go around in pairs). There are two possible ways of dealing with this, I will show you the commonest one first and then a suitable (and indeed acceptable) alternative next.
The commonest method is this one:
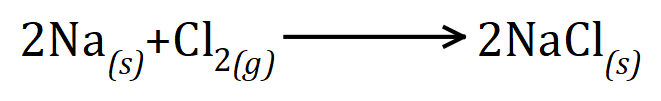
Taking into account chlorine is need for partnership we simply double up the amounts so that in fact two atoms of sodium will react with one diatomic chlorine molecule to form two units of sodium chloride (I deliberately said units not molecules because sodium chloride is in fact ionic and individual units do not in fact exist as sodium chloride forms a giant ionic lattice).
It is acceptable to show that the fact that chlorine is diatomic that that we only use one atom by regarding it as "half a molecule" but generally the first example is the most expected, particularly at GCSE level.
Balancing equations can become quite tricky when we start looking at larger atoms or groups of atoms, for example in the reaction between aluminium and chlorine:
"aluminium plus chlorine gives aluminium chloride"
But symbolically (yes, there is an error here...read on):

Straight away we can see that we have a problem, because we have two chlorine atoms on the left-hand side but three on the right. We cannot make extra chlorine atoms, they have to come from somewhere. Now the alternative that I showed you above would be very easy to implement here because if you study the equation you will quickly work out that to produce three chlorine atoms we need one and a half chlorine molecules, so if we prefix the chlorine gas in the equation with the number "1 1/2" we in fact end up with the correct equation, however it is customary to default to the first method and where fractions are involved we usually multiply up to remove them. So we would double the aluminium, triple the chlorine and double the product aluminium chloride as shown.
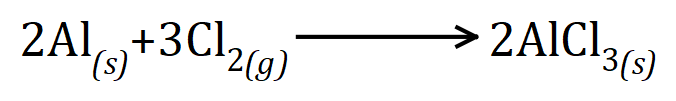
More on >> Balancing Equations <<