Effect of Concentration Change
The effect of changes in concentration on either side of the equilibrium are compensated for by a shift in the direction which reduces the concentration which is being raised. An example will hopefully make this a little bit clearer. Perhaps the most famous method for producing Ammonia gas is the Haber process, a high-pressure/high-temperature reaction between Hydrogen and Nitrogen, according to the equation:
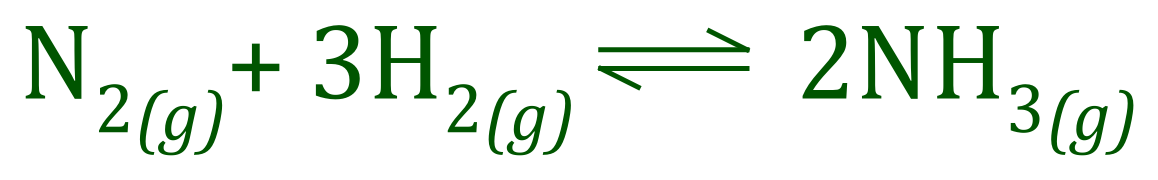
This reaction will be conducted at a very high temperature, in the 400° C area and under high pressure of around about 200 atm. Unless the Ammonia is "tapped off" and unused Hydrogen/Nitrogen re-entered into the system, an equilibrium will be set up as shown above.
What do you think might happen if we increase the concentration of Ammonia (product) during the Haber process? Well, Le Chatelier's principle states that the system will respond to try to negate change, so an increase in the concentration of Ammonia will favour the reverse reaction and the production of Nitrogen and Hydrogen.
Of course, during this particular industrial process we want to produce Ammonia so it is in our interests to favour the forward direction, so this is done by regularly removing produced Ammonia whereupon the system will respond to the change by favouring the production of more Ammonia.