Effect of Temperature Change
First of all take a look at temperature. All reversible reactions are temperature dependent, that is they to give out energy or they take it in and this is dependent on the direction. If a reversible reaction for example is endothermic in one direction it will be exothermic in the other. If you apply heat energy to an exothermic reaction, the principle states that the system will attempt to reverse the direction of the reaction so as to counteract the change, in other words it will try to favour the endothermic process.
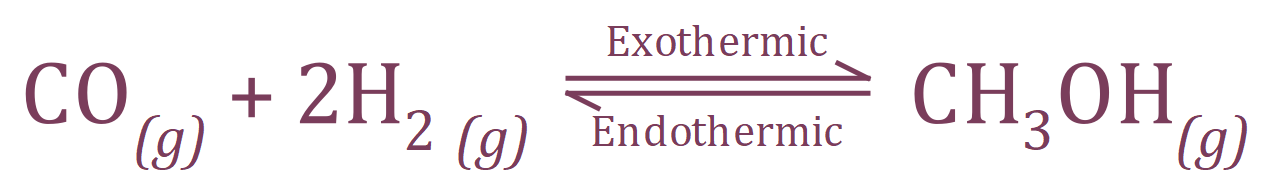
In the reaction above between Carbon Monoxide and Hydrogen, producing Methanol, the forward reaction is exothermic therefore the reverse reaction must be endothermic. This is shown in the above equation.If we increase the temperature of the system, which way will the equilibrium be shifted?. Well if you think about it, increasing the temperature is supplying thermal energy and the forward reaction is attempting to release thermal energy so these compete with each other. The reverse reaction is endothermic so it will absorb thermal energy, therefore in this case the equilibrium will shift to the left and favour the production of Carbon Monoxide and Hydrogen.
Understandably then, if we cool the system down, this will go against the endothermic process which is already trying to take thermal energy in, so the system is going to reverse the attempt to cool things down by increasing the thermal energy output, in other words favouring the exothermic process and shifting the equilibrium to the right.