Group 8/0 - The Noble/Inert Gases
Q1. What are the elements in group 8/0 of the periodic table commonly known as (two names)?
These elements are known as the Noble Gases, or less commonly now the Inert Gases. They are less commonly referred to as Inert as compounds of Xenon and Krypton have been made although this has been done under forced extreme conditions. In some periodic tables you may also see this referred to as Group 18.
Q2. What happens to the boiling points of the inert gases as we move down the group from Helium to Oganesson? **
As we move down the group, we see the boiling points steadily increase. This is because more energy is required to be put into "the system" to break the interatomic/intermolecular bonding between atoms of the elements. The larger number of electrons means that a larger temporary dipole can be set up between adjacent atoms which will produce a stronger interatomic bond, therefore requiring more energy to disrupt it.
Q3. The boiling point of the inert gas Xenon is -108°C. Given this information predict what state the inert gas Krypton would be at room temperature and explain your reasoning.
Xenon is further down the group than Krypton, therefore the temporary interatomic/intermolecular forces in Xenon will be higher due to the fact that there are more electrons **. Given the fact that the Xenon, despite this, is a gas at room temperature this will tell you that Krypton must also be a gas at room temperature.
** There are several kinds of intermolecular forces (all are in the broad category called van der Waal’s forces). These include hydrogen bonding (the strongest), dipole-dipole (intermediate) forces, and London Dispersion forces.
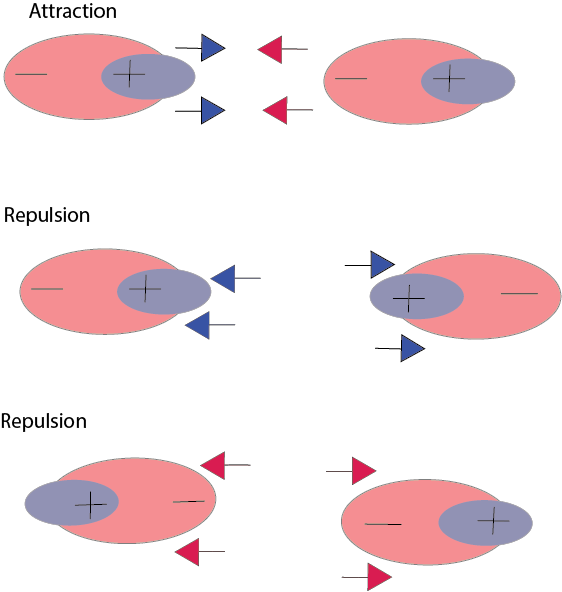
The weakest of the intermolecular forces is the London dispersion forces. These forces are created when the electron cloud around a molecule (or atom, in this case) can temporarily be displaced a bit, making negative charge move from one spot to one spot to another spot. This temporary dipole (small displacement of electric charge) can induce and attract dipoles in other molecules. The bigger the electron cloud, the easier it is for the electron density to shift and create those temporary dipoles. Thus, as you go down the column of noble gases, you are going to larger and larger sizes, therefore larger and larger London dispersion forces.
It’s these intermolecular forces that determines the boiling point. The weaker the force, the easier it is to separate the atoms. So, you can boil it at a lower temperature when the forces are lower than when they are higher.
Back To >> Questions <<
Back To >> Group 8/0 The Noble/Inert Gases <<