Hydrogen Bonds
When hydrogen is attached to an electronegative atom like fluorine, oxygen or nitrogen, a polar bonding will result. Because of the electronegativity, the electrons in the bond will be more attracted to the electronegative atom than to the hydrogen atom. Therefore, hydrogen atom will get a positive charge partially, whereas the more electronegative atom will get a negative charge partially.
When two molecules having this charge separation are close by, there will be an attraction force between hydrogen and the negatively-charged atom.
This attraction is known as hydrogen bonding:
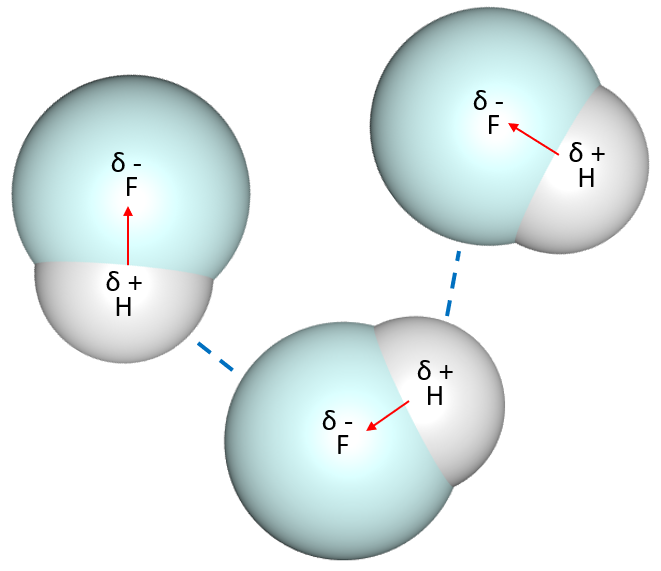
Hydrogen bonds are relatively stronger than other dipole interactions, and they determine the molecular behaviour. For example, water molecules have intermolecular hydrogen bonding. One water molecule can form four hydrogen bonds with another water molecule:
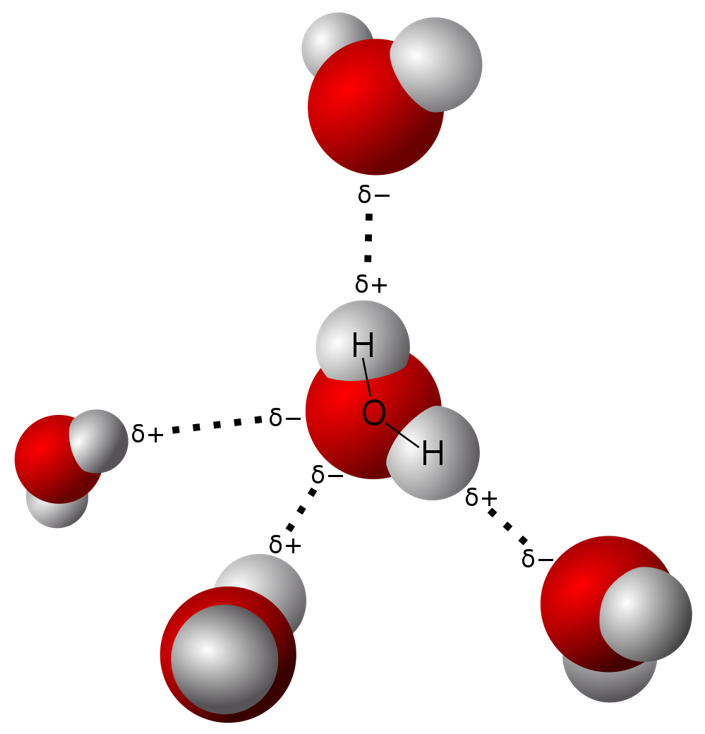
Since oxygen has two lone pairs, it can form two hydrogen bonds with positively charged hydrogen. Then the two water molecules can be known as a dimer. Each water molecule can bond with four other molecules because of the hydrogen bonding capability. This result in a higher boiling point for water, even though a water molecule has a low molecular weight.
Therefore, the energy needed to break the hydrogen bonds when they are going to the gaseous phase is high. Further, hydrogen bonds determine the crystal structure of ice. The unique arrangement of ice lattice helps it to float on water, hence protects the aquatic life in the winter period. Other than this, hydrogen bonding plays a vital role in biological systems.
The three-dimensional structure of proteins and DNA are solely based on hydrogen bonds. Hydrogen bonds can be destroyed by heating and mechanical forces.