Van der Waals Forces
In molecular physics, the van der Waals force, named after Dutch scientist Johannes Diderik van der Waals, is a distance-dependent interaction between atoms or molecules. Unlike ionic or covalent bonds, these attractions do not result from a chemical electronic bond; they are comparatively weak and therefore more susceptible to disturbance. The van der Waals force quickly vanishes at longer distances between interacting molecules.
https://en.wikipedia.org/wiki/Van_der_Waals_force - accessed 15.11.2019
To put the matter simply, molecules are held together by covalent or ionic bonds, that is the atoms become molecules by either sharing electrons or by donating them. In some cases one at some will donate all of the electrons required to make a bond, this is called a "coordinate" or "dative" bond. Weak forces of attraction exist between the molecules otherwise there would be nothing stopping the individual molecules from flying off into oblivion, and it is the strength of these forces that determine some physical properties such as melting and boiling points.
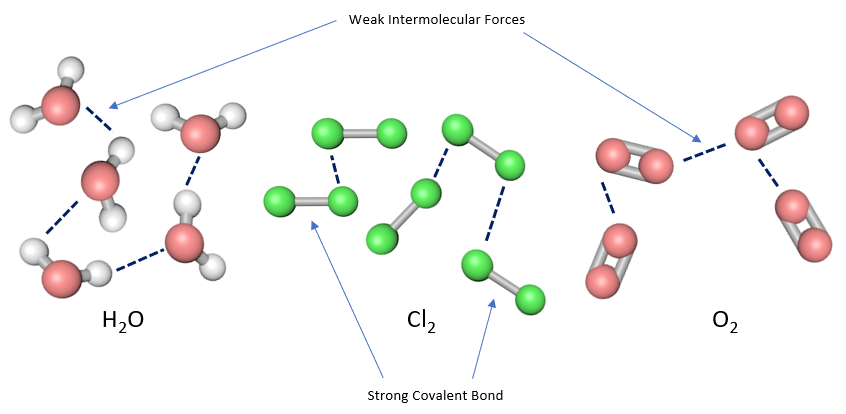
The dotted lines represent the weak intermolecular forces and the solid grey bars of course display the bonds between the atoms. When the substance melts or boils it is not the covalent bonds that are overcome, but is in fact the weak forces (the Van der Waals forces). How do these forces come into being in the first place?, We know the chemical bonds are made by the sharing or donating of electrons, as we said earlier but how do these weaker forces come into being?
Consider a single chlorine molecule:
An individual chlorine atom contains 17 electrons distributed across its energy shells. A chlorine molecule will therefore have 34 electrons in total, two of which will be shared to form the covalent bond which produces a chlorine molecule. It is beyond the scope of the intentions of this book to explain the electronic structure of the atom much beyond that of the "solar system" model that we are familiar with, however electrons do in fact occupy specific regions around the nucleus of the atom and it is perhaps easier to think of this as a cloud of negative charge.
If we consider the electrons therefore as a "cloud" and taking into account the fact that they are of course always moving around, it isn't unreasonable to assume that they won't always be equally distributed around the nucleus, and sometimes there may be more electrons on "one side" of the nucleus than on "the other":

A leap of faith is necessary to try to visualise this, but what you would end up with is a slight imbalance of electronic charge on opposing sides of the atom, producing a 'gradient' in the molecular bond. This could set up what is called an "instantaneous dipole" in the electronic environment of a neighbouring molecule, by electrostatically disturbing the electron cloud density in the second molecule.
If we think of this "instantaneous dipole" as the north and south poles of a bar magnet, we know that North attracts South, South attracts North and therefore there would be an instantaneous attraction between two atoms if there was an imbalance in the electronic charge. This is usually shown using the Greek symbol Delta with a positive or a negative to denote the charge type (or in this case deficit):
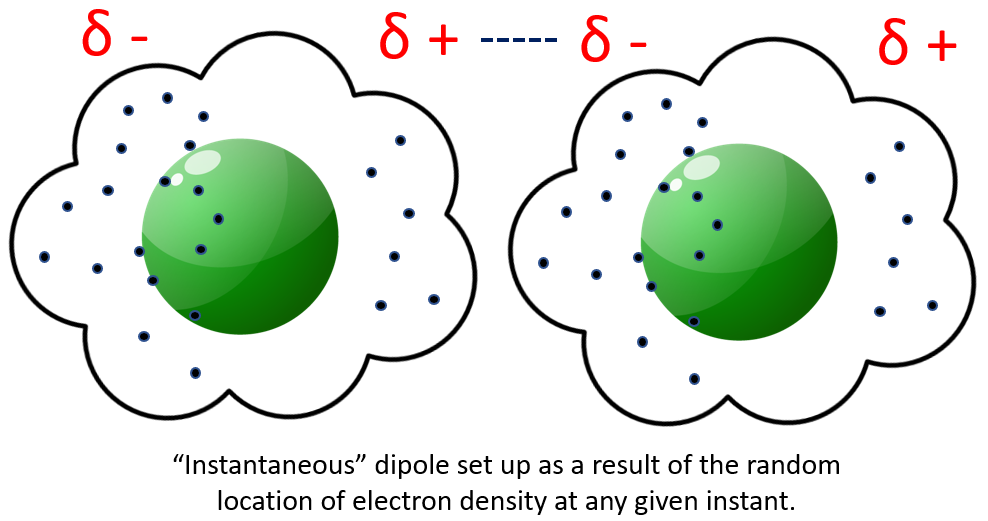
The dotted line shows the attraction between the positive and negative dipoles which would make the molecules cling together, albeit rather weakly. This force can disappear just as quickly as it appears, as aforementioned it is very weak and therefore quite easy to overcome.