Le Chatelier's Principle
Q1. A certain chemical reaction takes place according to the following equation:
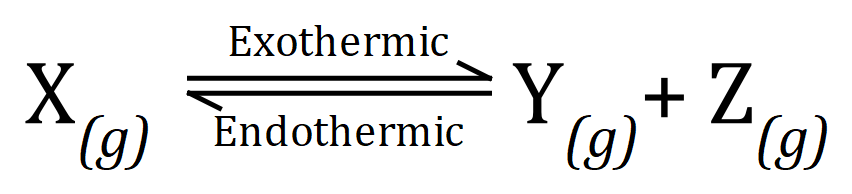
(a) Give an explanation as to what you believe the effect of increasing the temperature, or pressure, or concentrations of Y and Z and the equilibrium shown:
Temperature:
Pressure:
Concentration of X and Y:
Q2. A method of producing Ethanol is by the hydrolysis of Ethene (Ethylene) using steam (H2O) at very high temperatures (300oC) and pressures (60-70 atm):
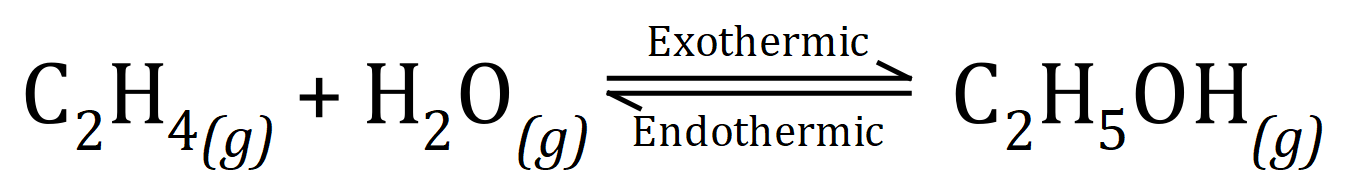
(a) This reaction takes place in a "closed" system, explaining a few lines what this statement actually means.
(b) What effect, if any, would an increase in temperature have on the yield of Ethanol?
(c) What effect, if any, would an increase in pressure have on the yield of Ethanol?
Go To >> Solutions <<
Back To >> Le Chatelier's Principle <<