Le Chatelier's Principle
Q1. A certain chemical reaction takes place according to the following equation:
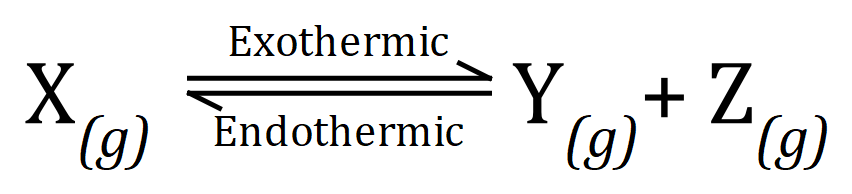
(a) Give an explanation as to what you believe the effect of increasing the temperature, or pressure, or concentrations of Y and Z and the equilibrium shown:
Temperature:
Pressure:
Concentration of X and Y:
Temperature: The reaction is exothermic in the forward direction and endothermic in the reverse direction. From Le Chatelier's principle an increase in temperature would cause the system to act to try to reverse the change, therefore the reverse direction, producing more of X would be favoured under the circumstances.
Pressure: Increasing the pressure would also favour the reverse reaction, on the right-hand side of the equation there are two molecules of "substance" whereas on the left-hand side there is only one. Increasing pressure could be counteracted by the system by favouring the direction of reaction which produces the fewest molecules, hence the reverse reaction (producing X) would be preferred.
Concentration of X and Y: Once again the reverse direction would preferred, for similar reasons to those explained in the increasing pressure. To reduce the concentration of Y and Z the system would attempt to make more X, favouring the reverse reaction once again.
Q2. A method of producing Ethanol is by the hydrolysis of Ethene (Ethylene) using steam (H2O) at very high temperatures (300oC) and pressures (60-70 atm):
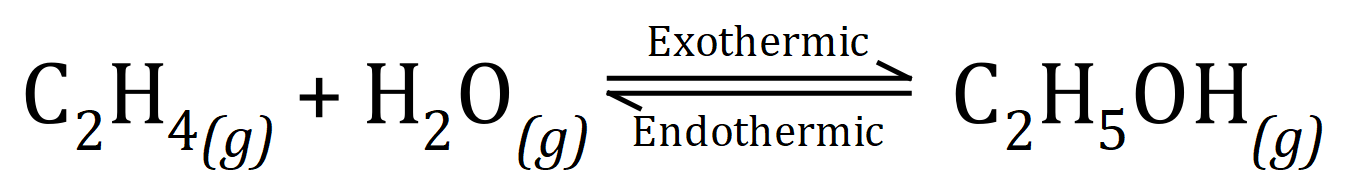
(a) This reaction takes place in a "closed" system, explaining a few lines what this statement actually means.
A closed system, is a system in which none of the reactants or products can escape, and nothing else can enter.
(b) What effect, if any, would an increase in temperature have on the yield of Ethanol?
Increasing the temperature would have the effect of decreasing the yield of Ethanol, because the system would respond to try to negate the change, in other words it would favour the endothermic reaction to absorb the extra input.
(c) What effect, if any, would an increase in pressure have on the yield of Ethanol?
An increase in pressure would push the equilibrium to the right, favouring the production of Ethanol. The system would attempt to reduce the effects of the increased pressure by favouring that "side" with the fewest molecules. You can see that the left-hand side of the equation has two molecules (moles) but the right-hand side only has one, the equilibrium therefore would be pushed to the right.
Back To >> Questions <<
Back To >> Le Chatelier's Principle <<