Proving Avogadro - Hydrogenation Of Propene
This is an interesting reaction, which supports the hypothesis that one mole of a gas at RTP occupies 24 dm3. Using a palladium catalyst, a suitable alkene (propene) or alkyne (ethyne) and hydrogen are mixed together whilst being passed over the catalyst. Ultimately the alkene or alkyne will be hydrogenated according to the chemical reactions shown below:
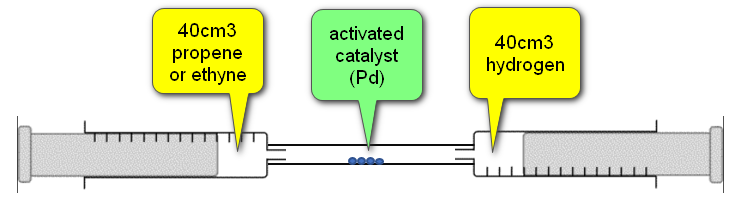
Point out that both syringes read 40 cm3, for a total of 80 cm3 of gas, and use the plungers to pass gas over the catalyst from one syringe to another. The catalyst tube will feel warm to the touch and the total volume of the gases will decrease. With 100% yield, the total volume will drop to 40 cm3, demonstrating Avogadro’s law:
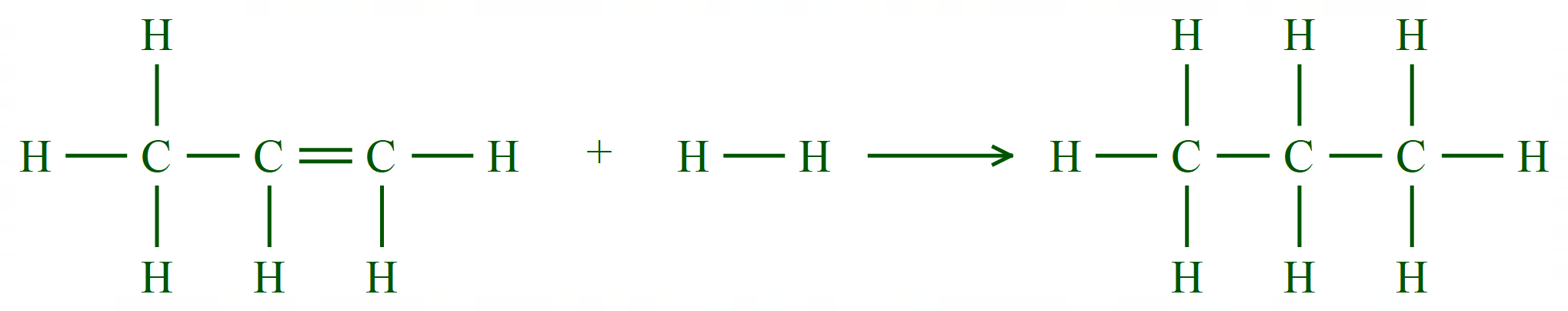
We started with 40 cm³ of Propene, and 40 cm³ of Hydrogen. As we are dealing with molar quantities, looking at the equation we can see that one mole of Propene will use one mole of Hydrogen to produce one mole of Propane. Therefore two moles of gas on the left-hand side will combine to produce one mole of gas on the right-hand side and this is why Avogadro's law is upheld. We start with a total of 80 cm³ of gas and with a 100% yield of Propane we would end up with 40 cm³ of gas in total which would all be Propane.
Bubble a few cm3 of product gas into approx 1 cm3 of 0.002 M acidified potassium manganate(VII) solution or 0.002 M bromine solution in a small sample vial, close the lid and shake. The product gas will not decolourise the contents, while repeating with the starting alkene will. This proves the absence of any double bonds which would allow the decolourisation of (say) bromine water by halogen addition across the double bond.
Finally, fit some silicone tubing to a glass Pasteur pipette and fix the other end of the tubing to the gas syringe. Burn off the contents by igniting the end of the Pasteur pipette as you expel the contents of the syringe. The starting alkene will produce a smoky flame, while the hydrogenated gas will burn cleanly. Again this is proving that Propane is not so "carbon rich" as Propene, and will therefore burn much more cleanly.