Rate Of Reaction
At this point in your studies you almost certainly know that chemical reactions are as a result of the making and breaking of chemical bonds and the breaking down of old compounds (reactants) and the formation of new compounds (products). This probably isn't a definitive explanation of a chemical reaction but it gives you an idea that chemicals (elements and compounds) under certain circumstances reacts to form new things by the recombination of elements and element groups.
What we are going to look at in this chapter is not so much the "why" but the "how quickly" these reactions take place and examine some of the conditions and participating factors that determine what we are going to refer to as "reaction rate" or "rate of reaction".
Some fairly slow reactions that are taking place all around us but that we probably don't give a second glance to do in our daily life are "rusting" which is the formation of iron oxide from iron and steel and the fermentation of sugar into alcohol.
Some medium rate reactions would include the dissolution of some metals in dilute acids, a favourite being the dissolution of magnesium in dilute hydrochloric acid (this in fact can be used as part of an experiment into looking at rate of reaction).
Examples of very fast reactions (which are in some textbooks regarded to be those reactions which take less than a millisecond to complete) could be explosions. The explosive combustion of petrol mixed with air inside an internal combustion engine producing various oxides of carbon, and water is an everyday example that we take for granted.
So we have a couple of questions, first of all why do some reactions happen quickly whilst others don't? What factors determine how quickly a reaction will take place? And finally how can we measure the rate of a reaction?
When a chemical reaction takes place, reactant particles have to:
- Come into contact with each other, they must collide with each other,
- Possess sufficient energy for the reaction to take place.
- Come into contact with each other with the correct orientation.
Some particulate collisions can take place without a reaction if they do not possess the required energy and / or orientation.
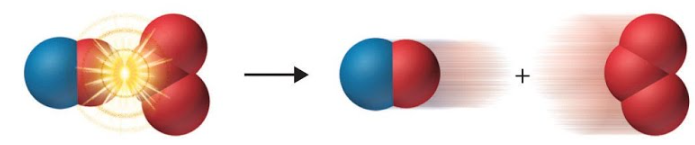
Consider the reaction between Nitric Oxide and Ozone (Trioxygen). In the first diagram we can see a collision occurring between an Ozone molecule and the oxygen atom in a Nitric Oxide molecule. This collision does not have the correct orientation for a reaction to take place and so therefore the reactant molecules simply bounce off each other.
The situation is quite different below:
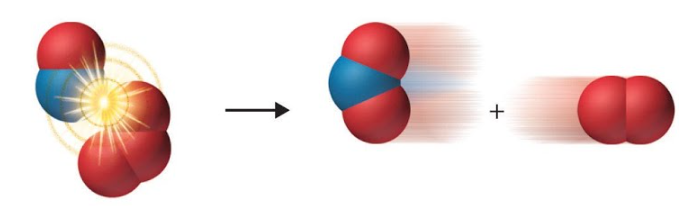
In this second example the collision point is between the Oxygen atom of the Nitric Oxide molecule and one of the Oxygen atoms present in Ozone, this is the correct orientation and coupled with the correct amount of energy will result in the Oxygen atom coming away from the Ozone molecule and bonding with the Nitrogen of the Nitric Oxide molecule, producing Nitrogen Dioxide and Oxygen.
If we consider the fact that not all collisions are going to be successful, what factors can you think of that may be the cause of this? Well, let us suppose that out of every 20 collisions, only one of them is successful. This isn't a particularly effective reaction but it serves a purpose. If we have 20 collisions per second this would tell us that we would only get one successful reaction/collision per second. What we actually want is more successful collisions, so the most obvious way to do this is to try to make the overall number of collisions increase. Let us say that we did something to make the collision rate increase to 100 collisions per second, now we would have five successful collisions each second, in other words we have increased the rate of reaction five fold.
Okay, we have decided that we need to make the number of collisions increase, but how can we do this?
- Increase the concentration of the reactants, the more particles we have per unit volume the more likely there is to be a successful collision.
- Increase the pressure of the system if we are talking about a gas state reaction, this will have the same effect as increasing the concentration because it will force the gas particles closer together so there will be more particles per unit volume.
- Increase the temperature of the reaction, increasing the temperature will give the particles more kinetic energy and so they should in theory collide with each other harder, thus ensuring that the rate of successful collisions improves.
- Increase the surface area of the reactants, thereby enabling more collisions (across a larger area to work on) and increasing the probability of a successful collision.
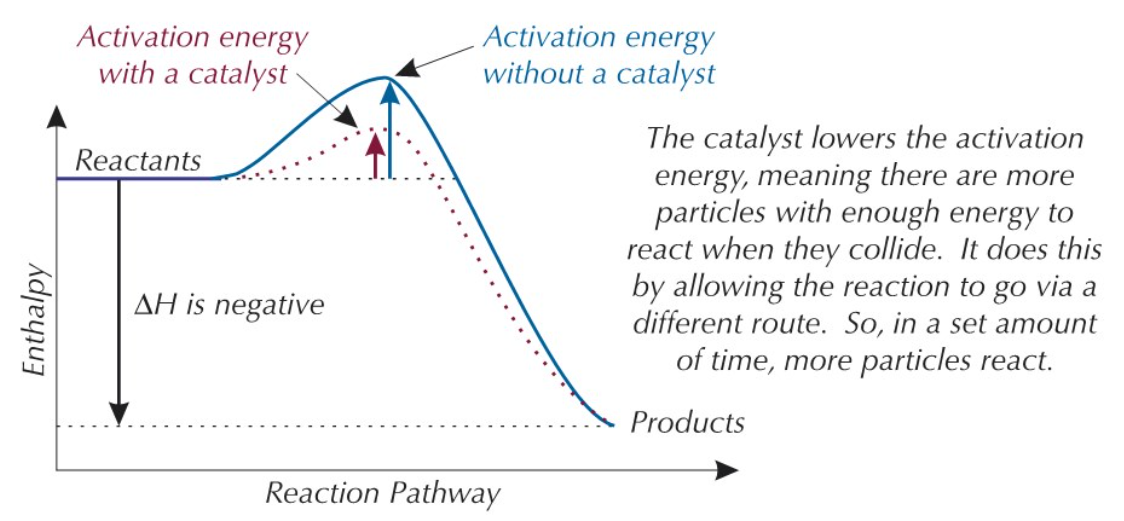
- Where applicable, introduce a catalyst to the reaction.
In the final bullet point, we introduce the concept of a "catalyst".
A catalyst is defined as:
"A substance which can speed up the rate of reaction without being (chemically) changed or used up in the reaction".
It is interesting because the resource from which I took this definition does not include the word "chemically" so I decided to include it myself. The reason for this is that a catalyst can be physically changed during the reaction, as long as it is not chemically changed otherwise it would become a reactant, or part of the product and could not then accurately be described as a catalyst. Some catalysts can actually be converted into products or chemically changed in some way, but this can only take place transiently, the catalyst must be transformed back at some point so that it does not have to be regarded as a reactant or a product.
So how did catalysts actually work?