Measuring Rate Of Reaction
From the bulleted responses previously, you will notice that three of them are highlighted in red. These are the examples that you will probably do as practical activities in the class and could face examination questions based on these practical activities, given appropriate values to work with.
For example increase in the concentration of the reactants, you may be given several different strengths/concentrations of hydrochloric acid and asked to compare the rate of evolution of hydrogen gas keeping the mass of (for example) magnesium metal constant. In this case you should notice that the higher the concentration/strength of the acid, the faster the reaction will occur.
Similarly you may be given one concentration of acid, but at several temperatures and you will notice that the higher the temperature of the acid, the faster the evolution of hydrogen gas. In both of these cases you have increased the frequency of particulate collisions and therefore will have sped up the reaction rate.
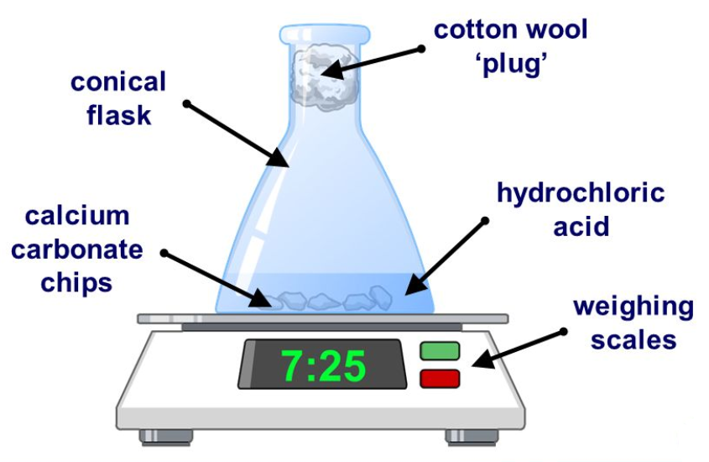
Increasing the surface area of the reactants is an experiment which you may conduct using Marble Chips and dilute Hydrochloric Acid. You will be given several sizes of marble chip ranging from large chunks to fine powder. Keeping the concentration of the acid constant you should notice that the evolution of (in this case) Carbon Dioxide gas increases in rate as the size of the particles decreases.
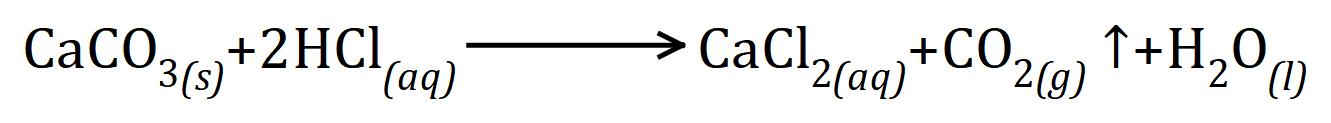
The large chips may bubble very slowly, the medium-size chips will bubble a little bit faster but the powder will bubble very rapidly and care must be taken so that this acid does not shoot out of the top of the flask, such is the speed and voracity of the reaction when you massively increase the surface area of the reactant and therefore increase the rate of successful particle collisions. The increase in successful particle collisions will produce more carbon dioxide in unit time, as a result the mass display on the balance will decrease more rapidly according to the increased rate of reaction.
There are a few ways to measure the rate of reaction, at this level you will probably use the Calcium Carbonate and Acid method as above or the production and measurement of the volume of Hydrogen using Magnesium and Acid. There is a third method, equally as fascinating as the other two although the chemistry behind it is slightly more complicated. The formation of a sulphur precipitate which slowly renders a transparent solution opaque, and the measurement of the rate at which this happens.
Sodium Thiosulphate solution reacts with Hydrochloric Acid to produce a precipitate of elemental Sulphur, Sodium Chloride solution, Sulphur Dioxide and Water according to the following equation:
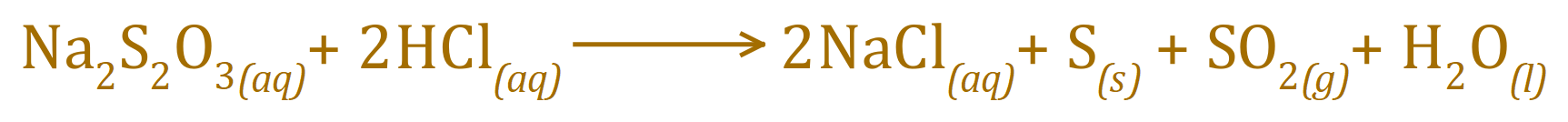


The rate at which the cross disappears (top picture to bottom picture) is determined by the concentration of Sodium Thiosulphate solution used. The higher the concentration of Sodium Thiosulphate, the faster the cross will be obscured.
Actual results from an experiment conducted by myself (29.09.2017)
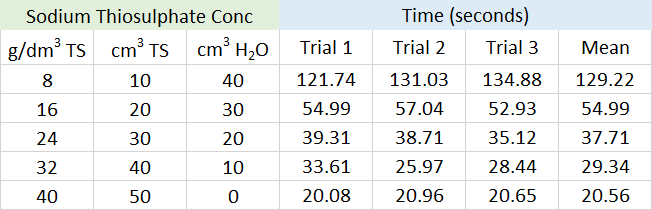
To calculate the rate of reaction at any given point would require a graphical plot of the data obtained, and a reading from a tangent to the curve at the time required. You will see that the rate of reation is always fastest at the start, but that whatever the concentration of reactant, it will slow to an eventual stop as the reactant is used up.
>> Questions <<