Sodium Thiosulphate and Hydrochloric Acid - Precipitation Reaction
Taking the data from the previous table and plotting it, it is quite clear that as the concentration of sodium thiosulphate increases, the time for "completion" (in other words the time it takes for the cross underneath the flask to disappear from view, which may not necessarily be at the point of completion because sulphur may still be being produced even after the cross has disappeared) drops considerably.
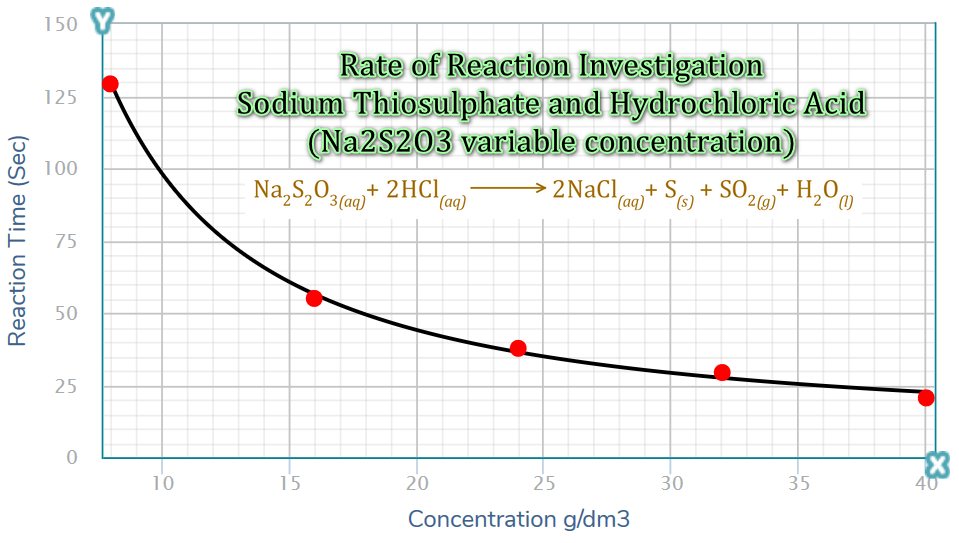
This can be quite simply explained using collision theory. In the higher concentrations of sodium thiosulphate there are more collisions between sodium thiosulphate molecules and hydrochloric acid molecules, therefore the probabilities of successful collisions leading to reactions will be higher.
As the concentration of sodium thiosulphate decreases, the collisions become more infrequent and so the probability of successful collisions reduces. If the rate of successful collisions reduces, the formation of elemental sulphur will take longer (This is probably easier to visualise if you look at the graph from right to left moving across the x-axis, as the concentration decreases the time for the reaction to "complete" increases because the number of collisions has decreased).