Specific Heat Capacity
As you would know from your studies of this subject, the specific heat capacity of a substance is a measure of how much energy it takes to change the temperature of a certain amount of that material by a certain number of degrees Celsius.
"The specific heat capacity is the amount of energy needed to raise the temperature of 1 kg of a substance by 1°C"
The questions in this section will concentrate on calculations involving specific heat capacity and, as is common throughout this book, some of the calculations will be requiring your mathematical skills to transpose the relevant equations in terms of the unknowns being sought. The amount of energy transferred to the thermal energy store of the substance through a given temperature change is linked to the specific heat capacity of the substance by the following equation:
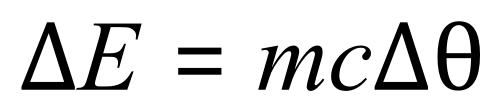
By now you should be mathematically skilled enough to realise that questions can be asked around how to calculate the mass the substance, the specific heat capacity and anything to do with the temperatures both start and finish as well as the energy change itself. This can be done by manipulating the above equation in terms of the unknown value that you're looking for. Let's take a look at some simpler examples before we start to dive into the trickier calculations.
>> Questions <<