Specific Heat Capacity
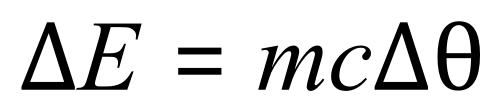
Q. Water has a specific heat capacity of 4200 J per kilogram degree Celsius. Calculate the amount of energy required to heat 5 kg of water from room temperature (20°C) to boiling point (100°C)
Q. Aluminium has a specific heat capacity of 900 J per kilogram degrees Celsius. A 10 kg block of aluminium is heated and 720 kJ of energy transferred into the aluminium's thermal energy store. Calculate the change in temperature from the start of the experiment to the end (note you do not need to know the start and the end temperatures, just the difference).
Q. A two tonne van is travelling at 30 m/s when a dog runs out in front of it. The driver jumps on the brake pedal and brings the van to a complete standstill. Given that the disc brakes have a combined mass of 25 kg and the material that they are made out of has a specific heat capacity of 420 J per kilogram degrees Celsius, calculate the change in temperature of the braking system during this incident.
Q. During the preparation of a meal, a lady heats up her deep fat fryer which contains 400g of cooking oil, reaching a temperature of 113oC. The oil is left to cool down later after the fryer has been used. Assuming that NO oil has been lost during the cooking process and that the start temperature is 113oC as stated, calculate the SHC of the cooking oil if the final temperature is 25oC and 70.4kJ of energy is released to the surroundings.
Go To >> Solutions <<
Back To >> Specific Heat Capacity <<