Specific Heat Capacity
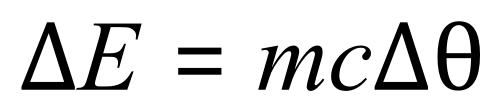
Q. Water has a specific heat capacity of 4200 J per kilogram degree Celsius. Calculate the amount of energy required to heat 5 kg of water from room temperature (20°C) to boiling point (100°C)
A. This is a straightforward "plug and go" type of question. You have been given values for the mass, the specific heat capacity and the temperature change. It is worthy of note at this point that it is always a good idea to write out the temperature change in parentheses, not to simply calculate it and use the final value. For example in this question we can see that the change in temperature Delta Theta is 100-20 which is 80 but my advice is to write 100-20 before you do the calculations. It is easy to try to do this calculation mentally, get them wrong and not realise that you have done so. You will then produce an answer which is wrong but you won't know why.
![]()
As the question hasn't asked for any conversions, this can be left as Joules as opposed to the possibility that you might be asked to quote your answer in kilojoules or megajoules. Always bear this in mind, read the question properly.
Q. Aluminium has a specific heat capacity of 900 J per kilogram degrees Celsius. A 10 kg block of aluminium is heated and 720 kJ of energy transferred into the aluminium's thermal energy store. Calculate the change in temperature from the start of the experiment to the end (note you do not need to know the start and the end temperatures, just the difference).
A. This is the first type of transposition question you could be asked for, you are given values for the energy, the specific heat capacity and the mass. What you don't know is the value for "Delta Theta" which is the temperature change. Take a look at what you do have, and make sure that the units are appropriately converted if they need to be. We have 720 kJ which is of course 720,000 J. This is our first conversion. The mass of the block is already stated in kilograms so that's okay and we have a specific it capacity quoted in the appropriate units as well. We are now in a position to transpose the equation in terms of "Delta Theta" and perform the calculation.
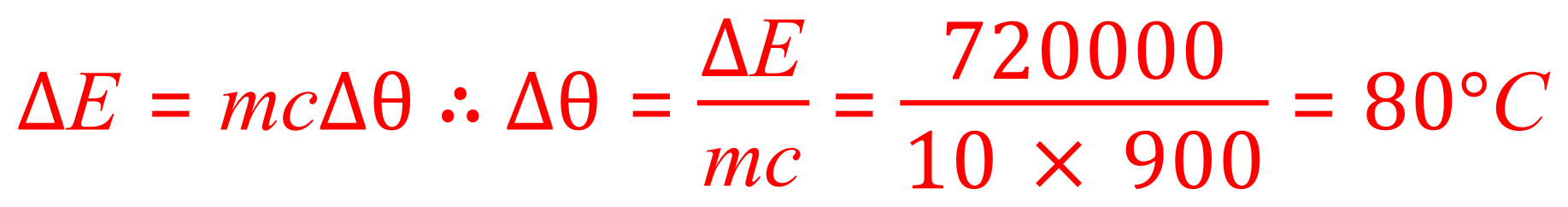
You may be given a question which, on the face of it, is a bit confusing to start with. The following question deals with energy transfer and the fact that we have to understand and remember the law of conservation of energy, that is energy cannot be created or destroyed but can only be transferred usefully or dissipated.
Q. A two tonne van is travelling at 30 m/s when a dog runs out in front of it. The driver jumps on the brake pedal and brings the van to a complete standstill. Given that the disc brakes have a combined mass of 25 kg and the material that they are made out of has a specific heat capacity of 420 J per kilogram degrees Celsius, calculate the change in temperature of the braking system during this incident.
A. There are a few things to consider here, first of all be told that the vehicle has a mass of 2 tonnes. We need a suitable conversion, and so we look at the fact that there are 1000 kg in a tonne. Our van therefore has a mass of 2000 kg. We are given the specific heat capacity of the material making up the braking system and we are told the speed at which the van was travelling. How much energy is being transferred here? Where is this energy coming from in the first place?
The van was initially moving and would therefore possess a certain amount of kinetic energy which we can calculate using the familiar equation.
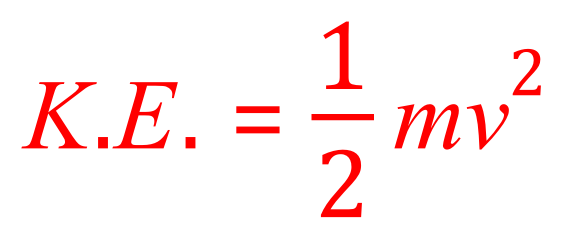
We know the mass of the van and we know it's velocity, if we apply the equation we can work out the kinetic energy that the van possessed in its kinetic energy store before the dog appeared.
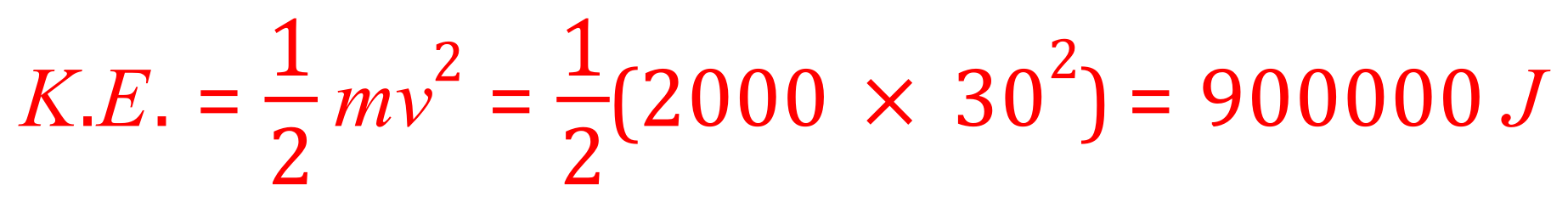
During the braking process all of this energy (assuming no losses through friction of the tyres on the road, air resistance or anything like that as we are not told to consider it in the question) will be transferred to the braking system. Looking at the equation for specific heat capacity we therefore now know the energy, the mass and the specific heat capacity of the material making of the braking system. What we don't know is the change in temperature "Delta Theta" which is what we will now calculate.
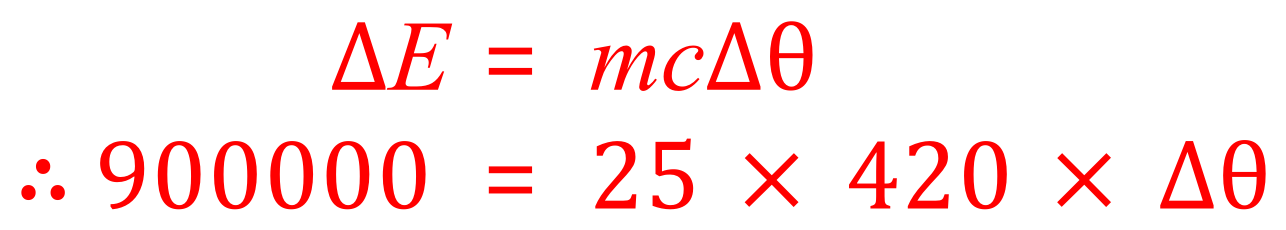
Remember at this point to put in the mass the braking system (25 kg) and not the mass of van! Rearranging equation now in terms of "Delta Theta" we have:
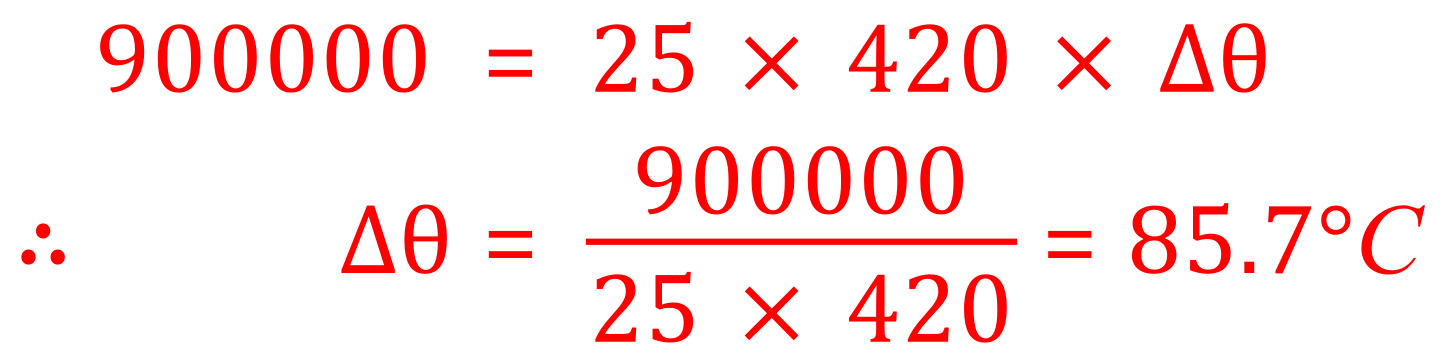
We can see that the temperature of the braking system increases quite dramatically to 85.7°C (to one decimal place) you will usually be told to what precision to quote you answer, under these circumstances it may be quite acceptable if you quoted your answer to 86°C.
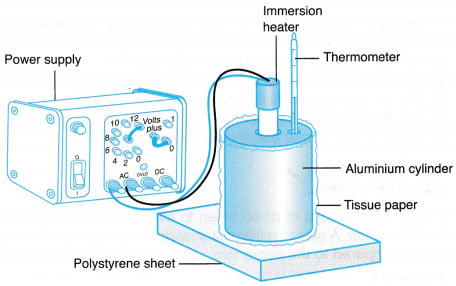
Investigating specific heat capacity is one of the required practical is in the GCSE physics syllabus (as of 2019) and is usually performed with aluminium block, a thermometer, a power supply, an immersion heater and an ammeter. The energy transferred into the block can be worked out by first working out the power of the heater (voltage times current) and then multiplying this power by the time 't' to work out the energy transferred. An alternative method is to use a "Joule meter" which will give a reading of energy transferred directly.
Q. During the preparation of a meal, a lady heats up her deep fat fryer which contains 400g of cooking oil, reaching a temperature of 113oC. The oil is left to cool down later after the fryer has been used. Assuming that NO oil has been lost during the cooking process and that the start temperature is 113oC as stated, calculate the SHC of the cooking oil if the final temperature is 25oC and 70.4kJ of energy is released to the surroundings.
A. In questions such as this one, always identify, and note, the data you are given, working on the premise that "if they've told you something, it's been done for a reason".
We are looking at a calculation for SHC and so the usual equation will be used:
![]()
Now collate what we have been given......ΔE = 70.4 kJ or 70400 J, m=400g and Δθ = (113-25) = 88oC. As we are only missing 'c', we need to transpose the equation so that 'c' is the subject:

Plug in your given, known values to arrive at a value for the SHC of the oil:
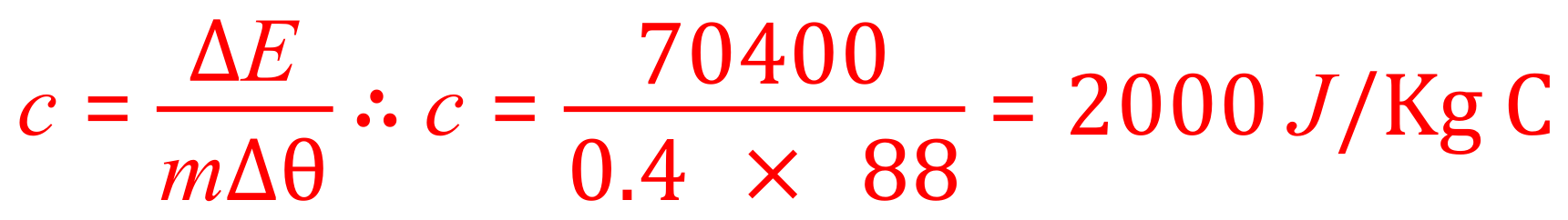
Back To >> Questions <<
Back To >> Specific Heat Capacity <<