States of Matter
Matter exists in three states (there is a fourth, plasma, however this is not discussed in GCSE level Physics) - Solid, Liquid and Gas.
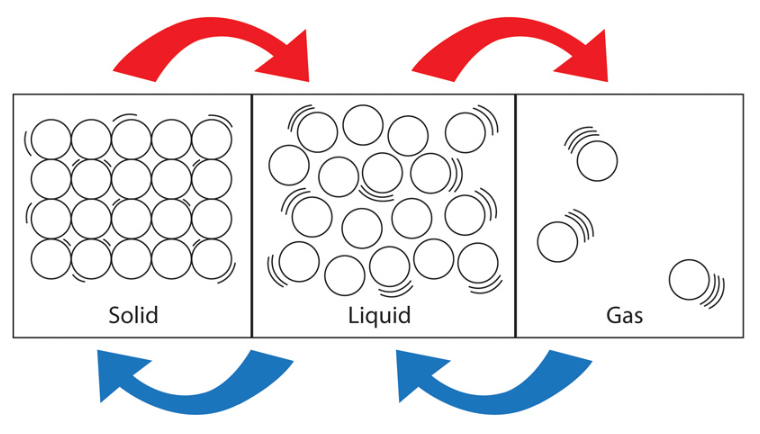
Solids - Have a fixed volume and shape. In solids the particles making up the material are locked in place in a fixed, regular arrangement. Strong forces of attraction hold the particles in place although they do have a certain amount of energy and as such are able to vibrate "in situ". This energy is not sufficient to allow them to break away from their fixed positions.
A solid will remain in shape irrespective of any container that you might put it in, for example if you placed a house brick into a shoe box, the brick would remain as it is, it would not take on the shape of the shoe box or spread out to try to fill it.
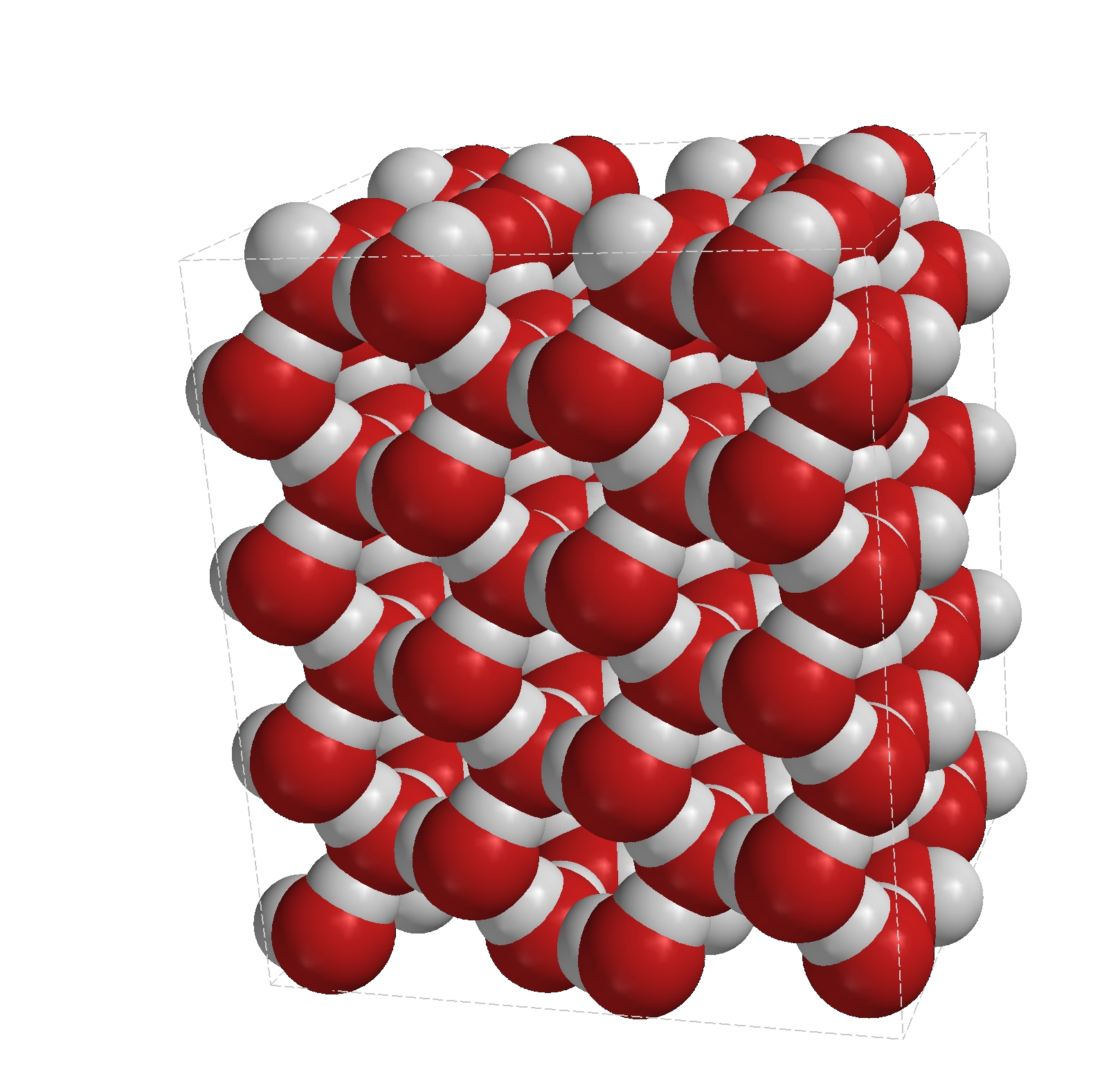
The image above is a model of a solid section of ice. "Solid water" where the molecules are arranged in a regular pattern. This is a particularly strong arrangement for ice and does in fact account for why ice has a greater volume than the amount of water it came from. Water molecules wrap around each other and take up as little space as possible because they are in constant contact with each other, but when water starts to freeze the ice crystals take up a regular (tetrahedral) pattern which naturally takes up more space and this is why ice is less dense than water.
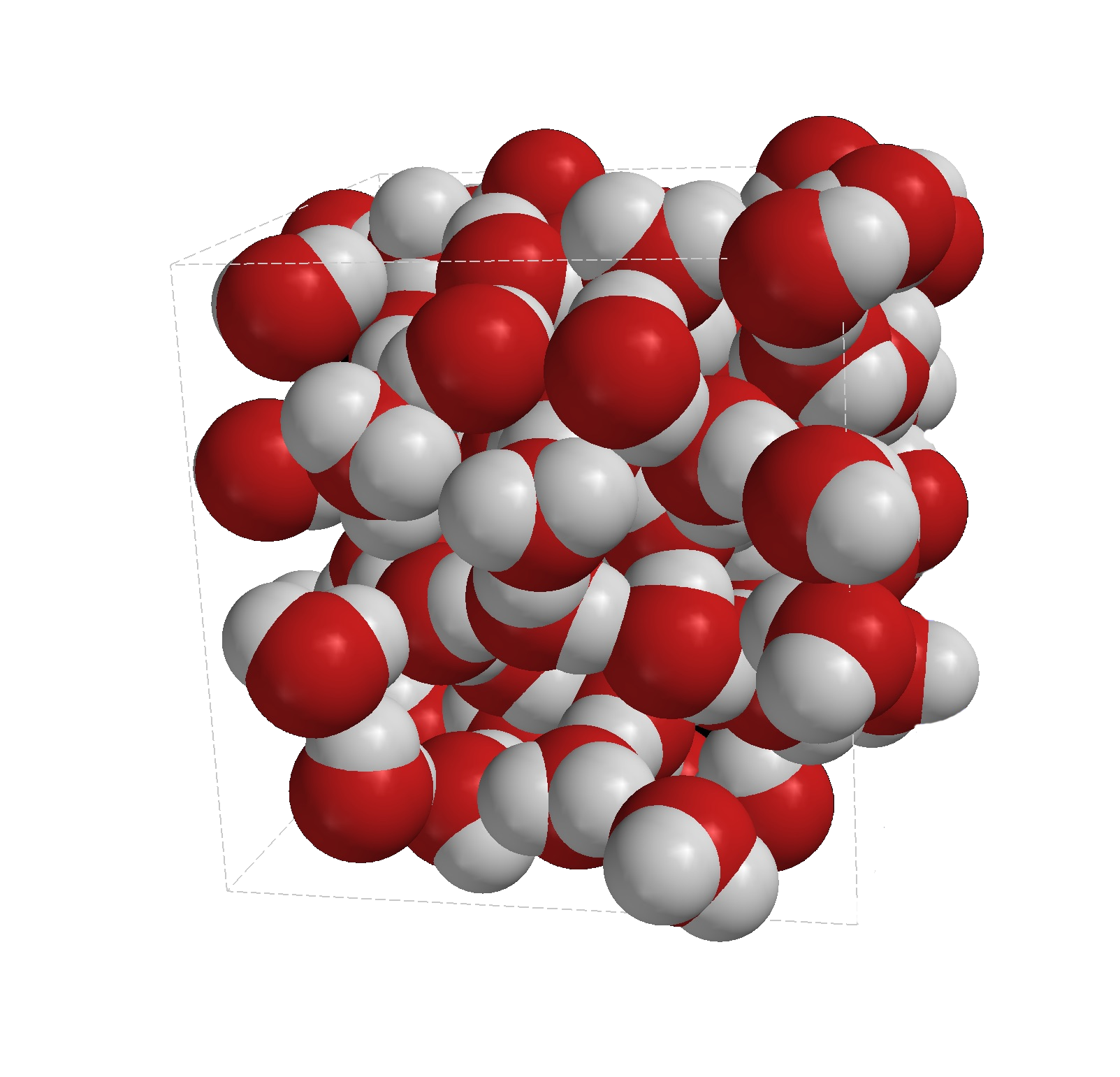
Continuing to use water as the model, when ice melts it turns back into liquid water, the molecules are no longer spread out in a regular structure and as such the volume that the water takes up is less than the volume the ice took up.
The arrangement of water molecules in the liquid form is much more irregular, as you can probably see from the picture. Although the molecules are in constant contact with each other they have sufficient energy not to be locked into place, but they are able to "slide" around each other and move around.
This ability to slide around each other and move around explains why liquid water will take the shape of the container that it is in.
If you wish to prove this to yourself, take an ice cube and put it into the bottom of a cup. You will see that the ice cube does not immediately take on the shape of the cup because it displays the properties of a solid (particles locked into place), however once the ice starts to melt you will see that it will reduce in volume and spread out to fill the base of the cup.
I should perhaps mention here that ice is an unusual example. The density of a substance is generally higher when it is in its solid form as the particles are closest together, becoming less and less dense as the solid passes through the liquid form and then enters the gaseous form.
Water is an unique example where the molecules "line up" to form a crystal structure when passing from the liquid to the solid phase (i.e. freezing) and as I said previously this is why ice is less dense than water and will float on it.
When the substance reaches its boiling point, we pass from the liquid phase to the gaseous phase. The particles have so much energy now that they are no longer held together by attractive forces. Continuing to use water as example, when water boils the molecules continue to vibrate but to such an extent that they can break apart from each other and no longer slide around each other as they did in the liquid phase. Water molecules with this sort of energy will evaporate into water vapour which we all know as steam.
Water molecules as steam now have so much energy that they can go anywhere they want to. Steam, as indeed all gases, will fill all of the available space so if your kettle is boiling in the kitchen and you leave it boiling, you will eventually find steam throughout the kitchen!
Throughout this section I've been talking about something called the "particle model" and it is a very useful model to help us explain the properties of the different states of matter.
To summarise, take a look at the table below:
|
State |
|
Density |
|
Comments |
|
Solids |
|
The density of a substance is generally highest in the solid form (however, this is not always the case) as the particles are closest together. |
|
As the particles making up solids are as close together as they can possibly get, solid materials are not compressible. |
|
|
|
|
|
|
|
Liquids |
|
The density of a liquid is generally less than that of the solid because the particles are slightly further apart, although they are still in contact with each other. |
|
Particles making up liquids can flow past each other, but they are still in contact which means they cannot be pushed any closer together. As a result of this liquids are generally not compressible and find many uses in hydraulic systems (for example car braking systems, and hydraulic lifting gear). |
|
|
|
|
|
|
|
Gases |
|
Gases are the least dense because the particles are no longer in constant contact with each other. |
|
The particles in a gas are very spread out, a gas can therefore be squashed into a smaller volume because you are just reducing the distance between the particles. |