Strong and Weak Acids
This can be quite a difficult topic to understand. Not all acids give up their hydrogen ions as readily as others (another way to say this is that some acids are better proton donors than others).
When acids are added two aqueous solution, they "dissociate" by ionisation to produce hydrogen ions, as we have already mentioned some do this better than others. For example the substance hydrogen chloride, which when dissolved in water produces hydrochloric acid ionises to form hydrogen ions and chloride ions:
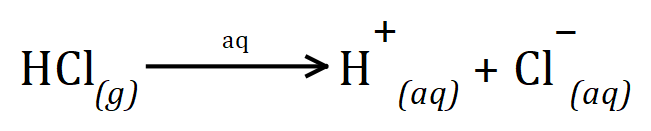
The strength of an acid is effectively the measure of how successfully the acid ionises to produce hydrogen ions in aqueous solution. You will already heard of strong acids such as sulphuric acid and nitric acid, both of these acids completely ionise in water but they produce different amounts of hydrogen ions as they do so.
(It should be noted here that acids capable of producing one hydrogen ion are regarded as monoprotic and acids capable of producing two hydrogen ions are diprotic. The chemistry behind how these acids ionise is more complicated than the scope of this particular text as, in the case of diprotic acids the two protons are not lost at the same time).
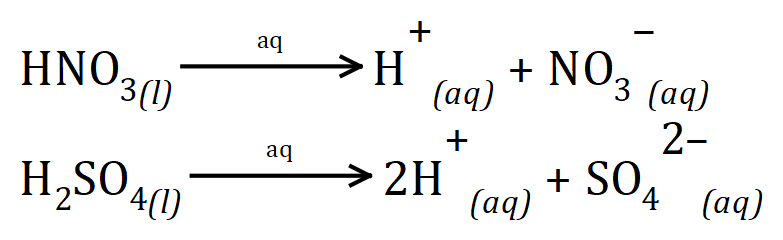
We can safely say (to keep this text at the level it is intended to be, i.e. GCSE) that strong acids dissociate completely in aqueous solutions whereas weak acids do not. We have seen some examples of strong acids such as hydrochloric acid, nitric acid and sulphuric acid and some of the weak acids that we will come into contact with (hopefully not literally) are ethanoic acid and carbonic acid.
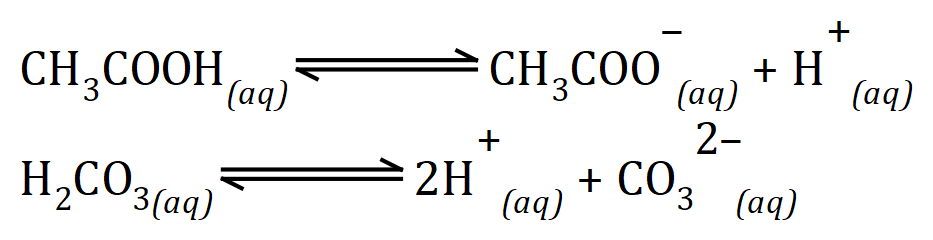
In fact it is quite rare to see carbonic acid shown in this way, generally you will find in textbooks you will read that where carbonic acid is intended to be produced it will be shown broken up into water and carbon dioxide.
This is the bit that students seem to struggle with, if two acids of the same strength are compared, then why don't they have the same pH?
In other words if we had a one molar solution of ethanoic acid and a one molar solution of nitric acid, why would the pH of the nitric acid be lower? Remember that the strength of an acid depends on the concentration of hydrogen ions in solution, therefore it makes sense that an acid which dissociates more will have a greater hydrogen ion concentration than one that does not.In addition to this, to complicate matters further students sometimes tend to forget that the pH scale is logarithmic, which means that an acid with a pH of 2 will have ten times the concentration of hydrogen ions than an acid with a pH of 3.
The difference between concentration and strength:
The concentration of acid is a measure of the total number of acid molecules that are dissolved in solution whereas the strength of the acid tells you what proportion of the acid actually ionises to produce hydrogen ions. As a strong acid will fully dissociate but a weak acid will not, you should now be able to understand that a strong acid and a weak acid at the same molarity will have the same concentration but different strengths.