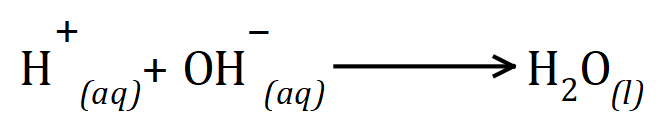Acids and Alkalis
Solutions can be regarded as acidic alkaline or neutral, depending on the pH. Just how acidic or basic a solution is can tell you a lot about its chemistry.The term pH is a measure of how acidic or alkaline a solution is, measured on a scale running from 0 to 14.
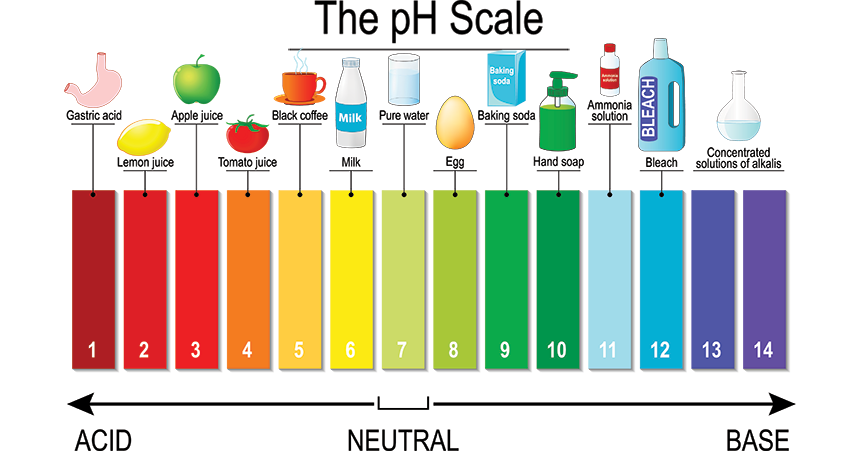
The diagram below gives you an idea of the acidity or alkalinity of some common household substances, anything below pH 7 is regarded as acidic and anything above pH 7 is regarded as alkaline.
pH is a measure of the hydrogen ion concentration of the solution, acidic solutions contain hydrogen ions H+ and alkaline solutions contain hydroxide ions OH-.
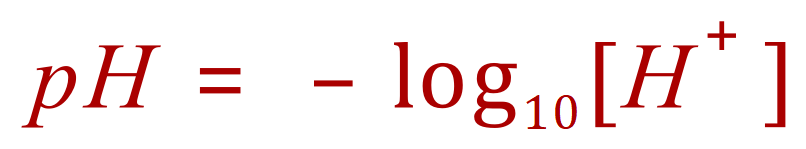
The way to calculate the hydrogen ion concentration from its pH therefore is to reverse the sign and take the anti-log to the base 10.
Q. An acidic solution has a pH of 3, what is its hydrogen ion concentration?
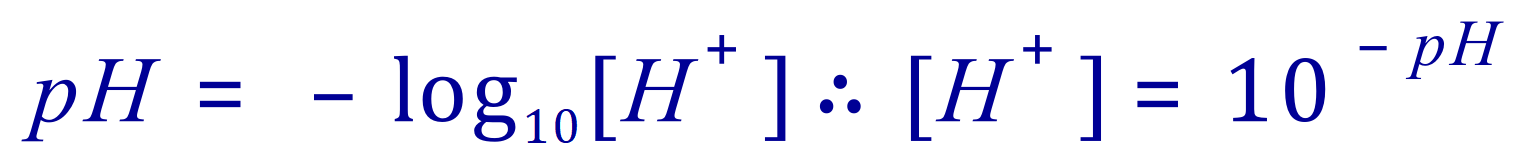
A. Given this, the [H+] in this case is 10-3 or 0.001 mol/dm3
From the diagram of the pH scale above, one way of testing the acidity or alkalinity of a substance is to look for a colour change of the substance known as a "universal indicator". Indicators are also used in titrations but these have to be specific in where they change, in other words an indicator which changes colour across a broad range such as universal indicator will not be able to tell us when the neutralisation has taken place so we need something a bit more specific such as Methyl Orange or Phenolphthalein.
So what exactly is a neutralisation reaction? What actually gets neutralised? Well, an acid will react with an alkali to form a salt and water, in fact this is one of the basic combination statements that you will learn in chemistry:
"acid plus base equals salt plus water"
You will come across others, especially in organic chemistry such as "acid plus alcohol equals ester plus water" but for the time being we will stick to the inorganic side of things.
During a neutralisation reaction, hydrogen ions (protons) will react with hydroxide ions to produce neutral molecules of water: