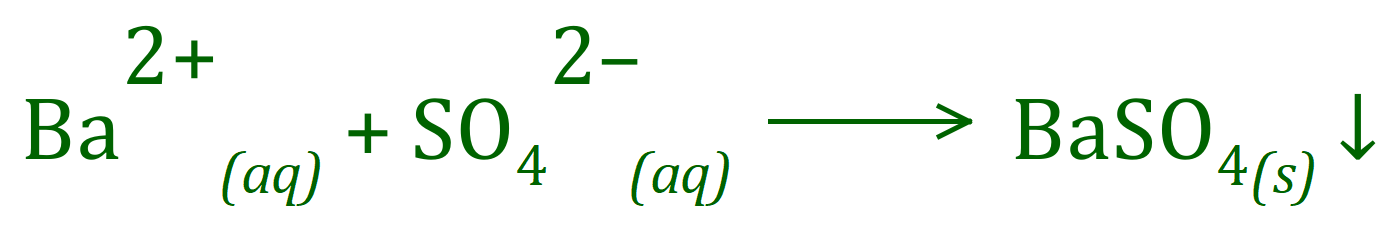Sulphates
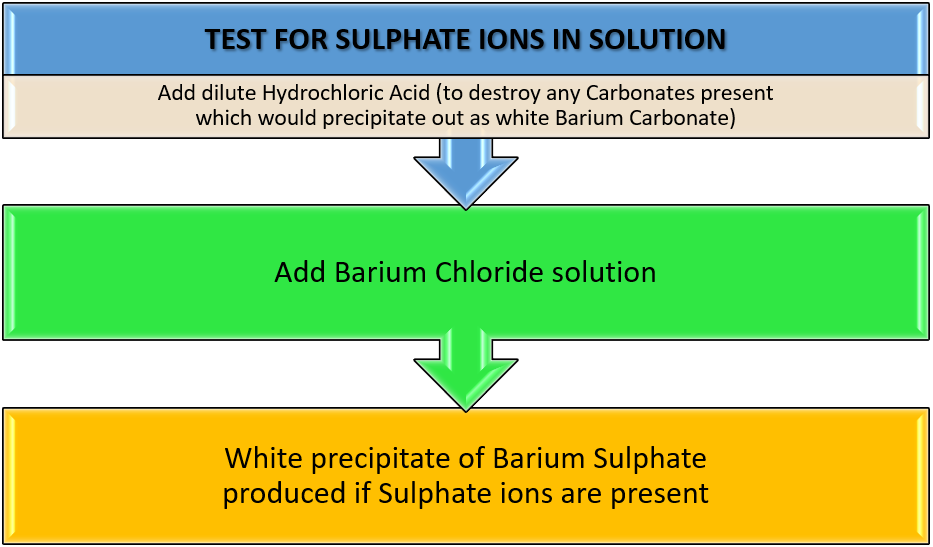
The test for Sulphate ions in solution is quite straightforward. Sulphate ions produce a dense white precipitate of solid Barium Sulphate when mixed with an aqueous solution of Barium Chloride. Before the test is carried out the solution is acidified with Nitric Acid to destroy any Carbonate ions that may be present, as these will also produce a white precipitate (of Barium Carbonate) which would confuse your results.