[A] Molar Concentrations In Solution
Q. What would be the concentration in moles per decimetre cubed of a solution containing 2.1 g of Sodium Hydrogen Carbonate in 250 cm³ of solution?
Na = 23, C = 12, O = 16, H = 1
The formula for sodium hydrogen carbonate is NaHCO3 and you will almost certainly be given this anyway. Given the relative atomic masses of the elements making up Sodium Hydrogen Carbonate, you can work out that the relative molecular mass would be 23+1+12+(3x16) = 84.
The amount that we are concerned with is in fact 2.1 g, but this is in 250 cm³. A "molar" solution would require that the volume is made up to one decimetre cubed, and this would require 4 times the amount of Sodium Hydrogen Carbonate, that is 2.1×4 = 8.4g.
The relative molecular mass of Sodium Hydrogen Carbonate is 84 as we have already seen, so 8.4 g would make a solution with a concentration of 0.1 mole (or 0.1 molar).
Q. A solution of dilute Sulphuric Acid has a concentration of 4.9 g dm-3 . What is its concentration in moles per decimetre cubed?
H = 1, S = 32, O = 16
We are given the relative atomic masses of the components of Sulphuric Acid, that is Hydrogen, Sulphur and Oxygen. We can work out that the relative molecular mass of Sulphuric Acid is 98 [(2x1)+32+(4x16)]. You should therefore now know that a molar solution of Sulphuric Acid would contain 98 g, but we are told that our solution contains only 4.9 g per decimetre cubed, this means that we have a concentration of:

Q. What would be the concentration in grams per cubic decimetre of Potassium Hydroxide solution with a concentration of 0.2 moles per cubic decimetre?
K = 39, O = 16, H = 1
Potassium Hydroxide has the relative molecular mass (or relative formula mass, if you like) of 39+16+1=56. Therefore a one molar solution would contain 56 g of potassium hydroxide dissolved in water and made up to 1 dm3. As we require a 0.2 molar solution, we multiply 56 x 0.2 to work out the number of grams of potassium hydroxide we would need to use:
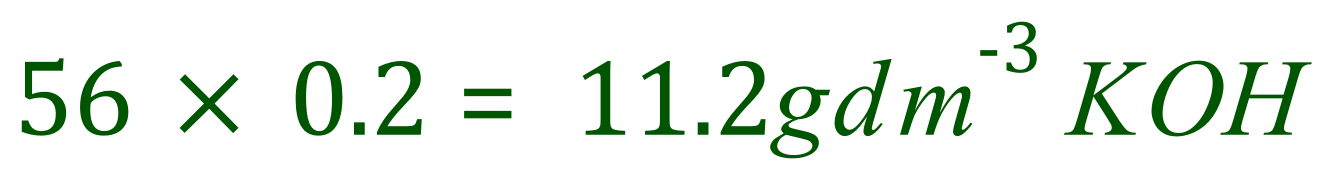
Q. What would be the mass of Sodium Carbonate dissolved in 100 cm³ of solution in order to get a concentration of 0.1 moles per decimetre cubed?
Na = 23, C = 12, O = 16
This question is fairly straightforward. We know that a molar solution of Sodium Carbonate would contain 106 g of the salt, dissolved in water and made up to one decimetre cubed. A 0.1 molar solution would therefore contain 1/10 of this, at 10.6 g and 100 cm³ of this solution would therefore contain a 1/10 of this, ie: 1.06g.
Back to >> Questions <<