[A] Stoichiometry (Volumetric Analysis )
Q1. During a neutralisation experiment, a student found that 10cm3 of Sulphuric Acid was required to neutralise 25cm3 of Calcium Hydroxide solution at a concentration of 0.2M, given this information and the balanced chemical equation for the reaction, calculate the concentration in moles per litre and grams per litre of the Sulphuric Acid.
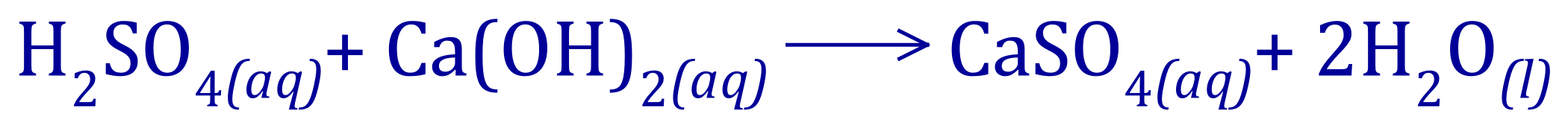
In these types of question, make a note of the information you have been given. We are told that the concentration of calcium hydroxide is 0.2 molar and that we used 25 cm³ of it, we therefore need to the calculate number of moles of calcium hydroxide in that volume.
If we have 0.2 moles in 1 dm cubed (1 L, 1000 cm³) of the calcium hydroxide solution, then in 25 cm³ we will have:
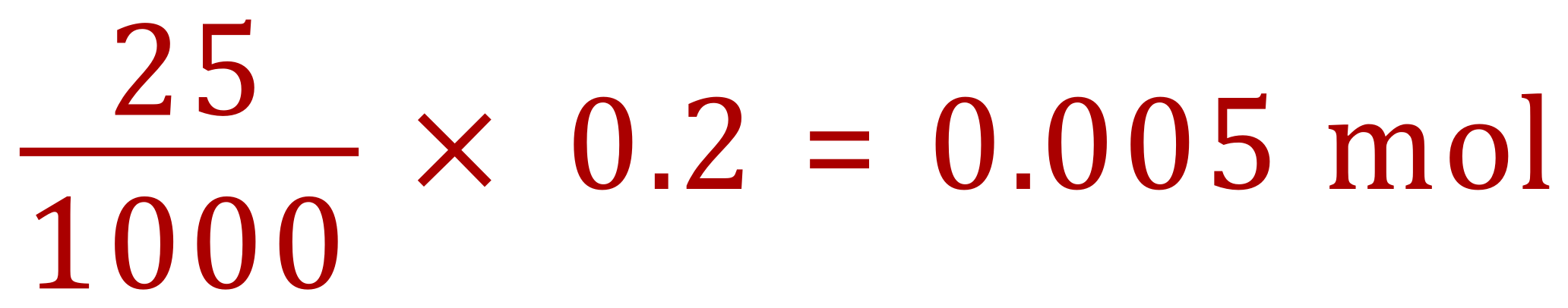
Now that we know the number of moles of Calcium Hydroxide, we need to look back at the balanced equation and from this we can see that the relationship between the acid and the base (the Hydroxide) is one-to-one, in other words one mole of Sulphuric Acid will react with one mole of Calcium Hydroxide to produce one mole of Calcium Sulphate and two moles of water.
From our previous calculation we know that we used 0.005 moles of Calcium Hydroxide, therefore we can conclude that we will also use 0.005 moles of Sulphuric Acid as, as we just said, the relationship is one-to-one.
We can now state that 10 cm³ of the sulphuric acid contains 0.005 moles, therefore 1000 cm³ (1 L, 1 dm cubed) will contain:
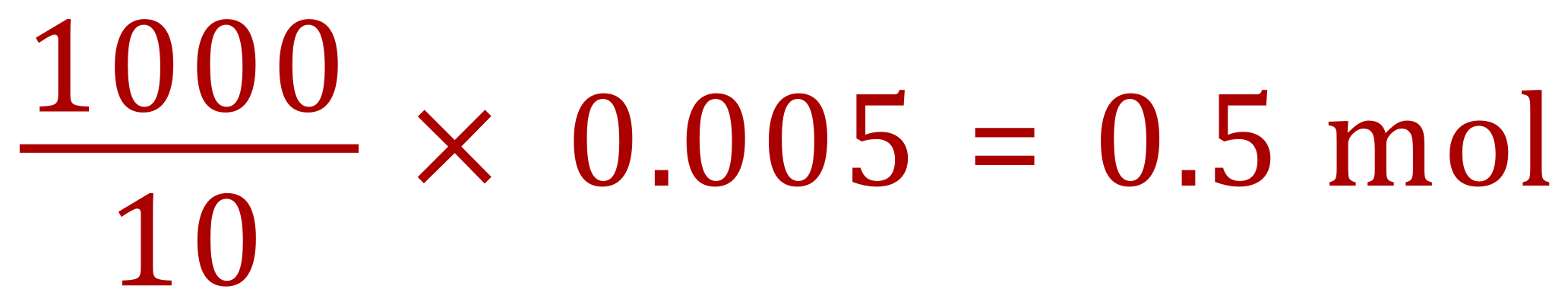
And this is the concentration of the acid, 0.5 molar. To complete the second part of the calculation (working out the grams per litre) requires us to know the relative formula mass of Sulphuric Acid. We can quickly work this out using a fairly basic periodic table as 98.
The concentration in grams per litre is therefore:
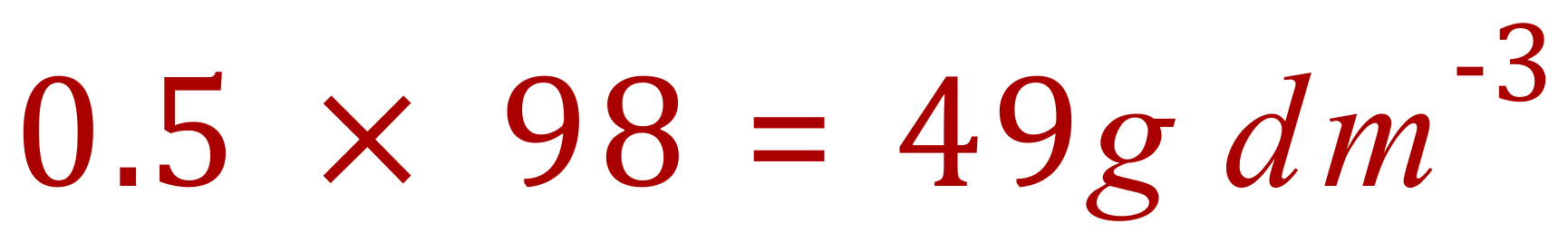
Q2. 25 cm³ of 0.1 molar Sodium Hydroxide solution required 23.5 cm³ of dilute Hydrochloric Acid for complete neutralisation. Calculate the concentration of the Sodium Hydroxide solution.The balanced equation for the reaction is given below:
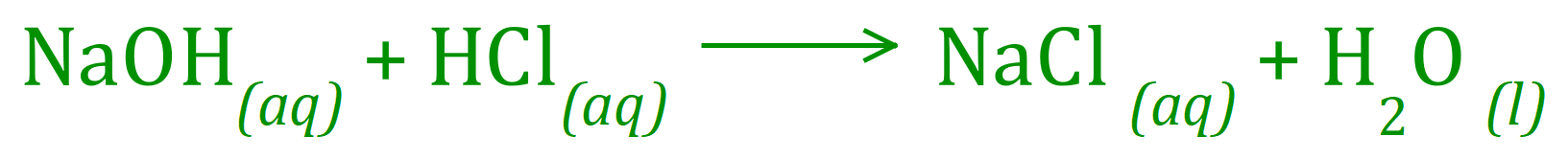
This is a simpler overall calculation compared to that in question one. You are told that the concentration of Sodium Hydroxide is 0.1 molar, in other words 0.1 moles per cubic decimetre/litre/thousand cubic centimetres.
The number of moles of Sodium Hydroxide in 25 cm³ of the solution would therefore be:
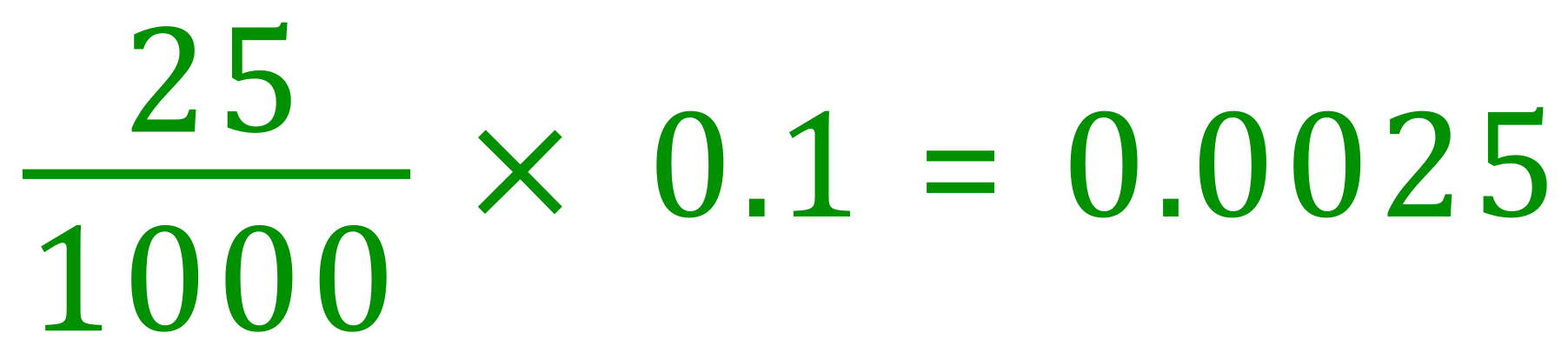
As before, now look at the balanced equation for the reaction and determine the ratio of Sodium Hydroxide to Hydrochloric Acid, it should be quite obvious that this is once again a one-to-one reaction in as much as one mole of Sodium Hydroxide requires one mole of Hydrochloric Acid to produce one mole of Sodium Chloride and one mole of Water.
We can therefore conclude that 0.0025 moles of Hydrochloric Acid were used in the neutralisation, and we are told that this was contained in 23.5 cm³ of the solution. The number of moles in 1 L of the solution (and therefore the concentration/molarity) would be therefore:
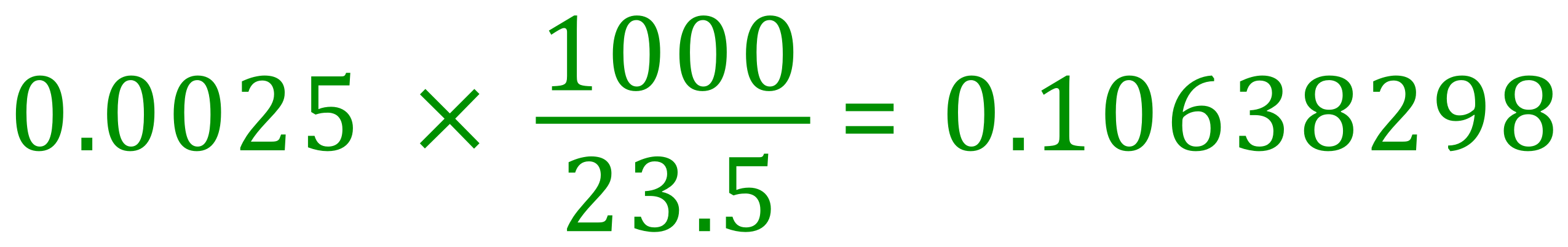
The concentration of the hydrochloric acid is therefore 0.106 moles per litre.
In both of the question so far, in arriving at the final concentration you have seen an expression containing an improper fraction of 1000 over something, you may be wondering where this comes from.
Assuming that you are okay with the first part of the equation, for example in the second question the establishment of 0.0025 moles from 25/1000 and then multiplication by 0.1, so here is how we arrive at the second expression.
We established in part two of the calculation that we would require the same number of moles of hydrochloric acid as we did sodium hydroxide but of course in the latter case, the concentration of the acid is as yet unknown, we need to calculate how many moles per litre given the fact that we have 0.0025 moles in 23.5 cm³.
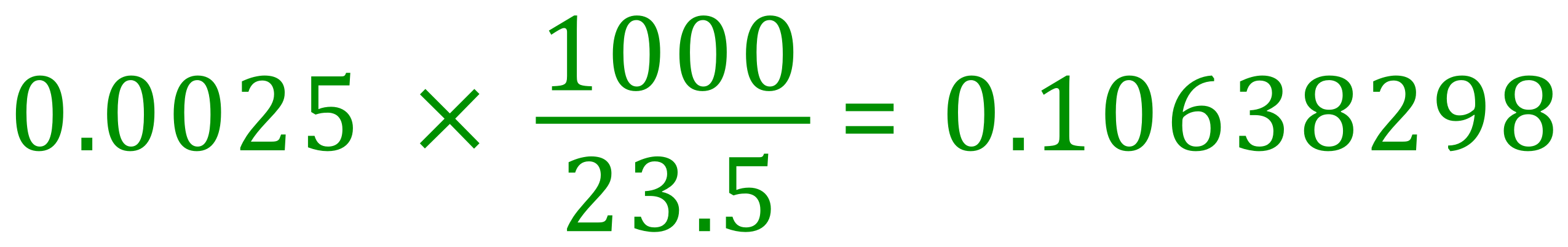
Repeated for convenience is the final step in the calculation. We know that there are 0.0025 moles in 23.5 cm³ so we need to divide by 23.5 cm³ to establish how many moles there would be in 1 cm3 and then multiply this value by thousand to establish the molarity in moles per litre. All I have done in essence is combine these two calculations in one by using a bulky looking improper fraction.

The reason that I felt the need to explain this in detail is that, in my experience, it is the part of the calculation which tends to trip students up.
Q3. 25 cm³ of Potassium Hydroxide solution of an unknown concentration was titrated with dilute Sulphuric Acid at the concentration of 0.05 moles per cubic decimetre. 20 cm³ of the acid was required to neutralise the alkali. Calculate the concentration of the Potassium Hydroxide solution in moles per cubic decimetre.
The balanced chemical reaction equation is this:

Once again, apply the rules and work out what you know from what you don't know. We are told that the concentration of Sulphuric Acid is 0.05 molar and that 20 cm³ of it was used in the neutralisation. We therefore calculate the number of moles of Sulphuric Acid that was involved in the neutralisation.
Moles of Sulphuric Acid:
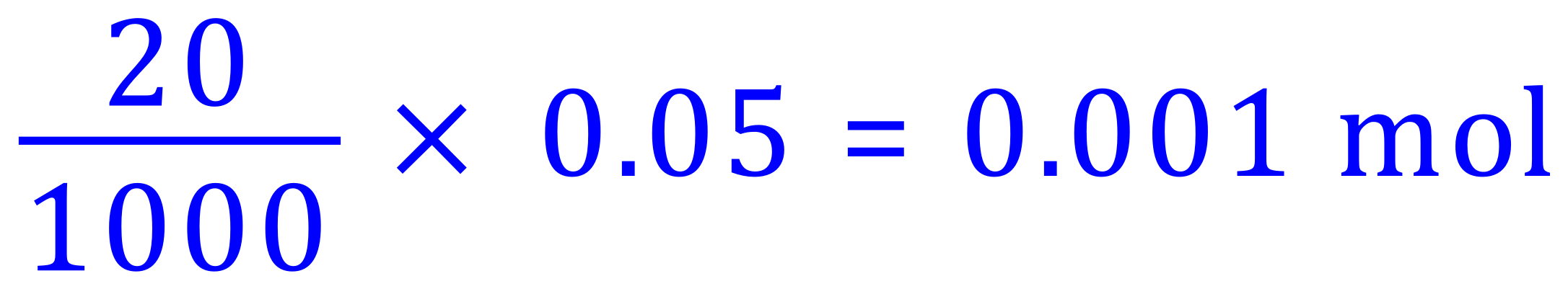
We have therefore used 0.001 moles of Sulphuric Acid in the neutralisation, but this time from the equation we can see that the ratio is in fact 2-to-1 in as much as we need 2 moles of Potassium Hydroxide to react with one mole of Sulphuric Acid.
You should by now be able to work out for yourself that if we have used 0.001 moles of Sulphuric Acid we will need 0.002 moles of Potassium Hydroxide because of the reacting ratio.
Moles of Potassium Hydroxide:
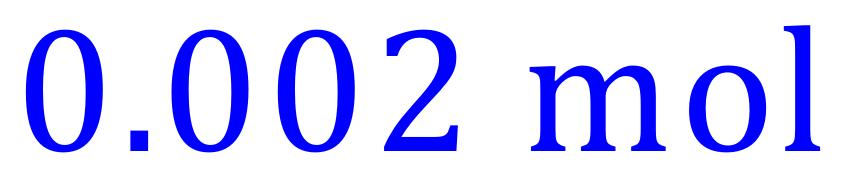
We are also told that this many moles of Potassium Hydroxide were contained in 25 cm³ of the solution, so we can work out the molarity of the Potassium Hydroxide solution thus:
Molarity of Potassium Hydroxide:
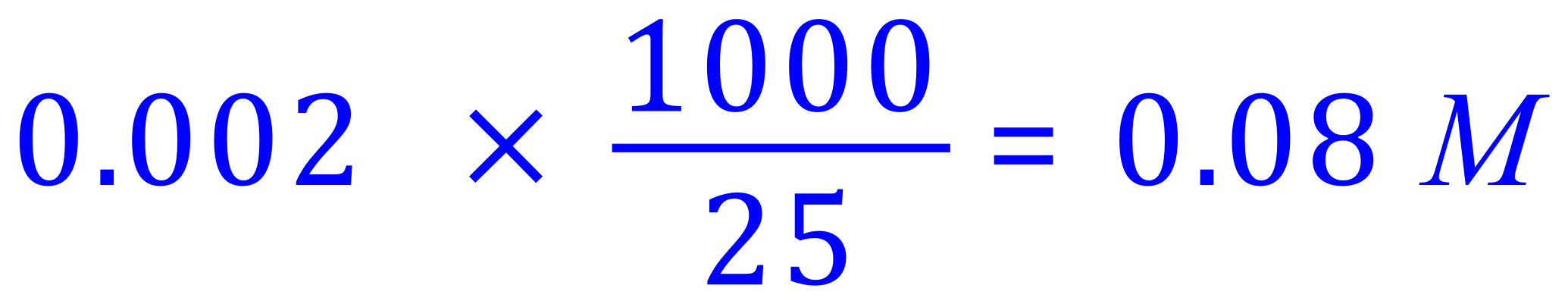
Back To >> Questions <<
Back To >> Calculations Regarding Concentrations of Solutions <<