{A) Stoichiometry (Volumetric Analysis )
Q1. During a neutralisation experiment, a student found that 10cm3 of Sulphuric Acid was required to neutralise 25cm3 of Calcium Hydroxide solution at a concentration of 0.2M, given this information and the balanced chemical equation for the reaction, calculate the concentration in moles per litre and grams per litre of the Sulphuric Acid.
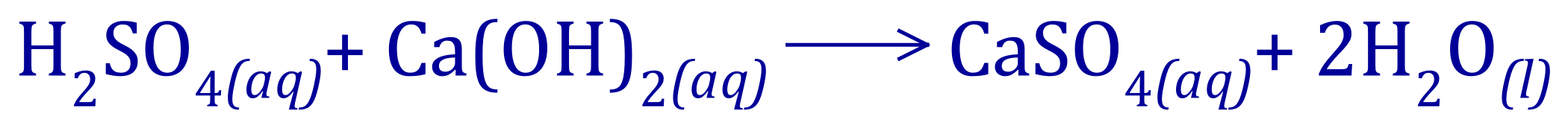
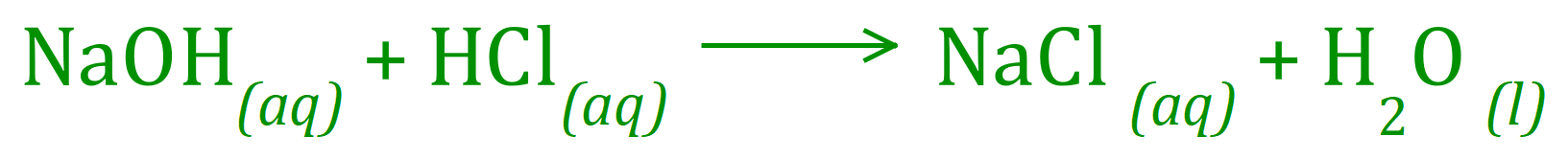
Q2. 25 cm³ of 0.1 molar Sodium Hydroxide solution required 23.5 cm³ of dilute Hydrochloric Acid for complete neutralisation. Calculate the concentration of the Sodium Hydroxide solution.The balanced equation for the reaction is given below:
Q3. 25 cm³ of Potassium Hydroxide solution of an unknown concentration was titrated with dilute Sulphuric Acid at the concentration of 0.05 moles per cubic decimetre. 20 cm³ of the acid was required to neutralise the alkali. Calculate the concentration of the Potassium Hydroxide solution in moles per cubic decimetre.
The balanced chemical reaction equation is this:

Go To >> Solutions <<
Back To >> Calculations Regarding Concentrations of Solutions <<