[A] Calculations Regarding Concentrations of Solutions
One of the required practicals involves volumetric analysis, an old name for what is now simply referred to as "titrations". Using this method you can work out the concentrations of a solution from the concentration of another solution that it reacts with, usually in neutralisation reactions where the endpoint (the point at which the reaction has completed) can be detected using an indicator of some sort, for example phenolphthalein. There are already a couple of worked examples of these on the appropriate page, if you are using the electronic version you may simply >> click here << to go directly to that page.
As you should already know, indicators are used to show when the endpoint of the neutralisation reaction has been reached, the aforementioned substance phenolphthalein changes from pink to colourless (it is colourless in acidic conditions and pink in alkaline conditions) or vice versa at the endpoint depending on which of the acid or base/alkali is being titrated against the other. Usually you will find that the acid is in the burette and the alkali is in the conical flask, but it does not have to be this way.
Q. 0.0350 dm3 of sodium hydroxide solution was put into a flask. The concentration of the sodium hydroxide solution was 0.500 molar. 0.0250 dm3 of hydrochloric acid was needed to neutralise it
(a) Calculate the number of moles of sodium hydroxide used
(b) How many moles of acid were needed to neutralise the sodium hydroxide?
(c) Calculate the concentration of the hydrochloric acid in moles per cubic decimetre.
A.These three-part questions are quite common and you may find that in some of them you will need to produce answers to earlier parts to be used in later parts, in some cases if you cannot answer the earlier part you will be given a specimen answer to work with to carry on with the later parts (this will almost certainly be incorrect with regard to the part of the question it was meant to answer but suitable for you to carry on with the question).
(a) We are told that we have 0.0350 cubic dm of 0.5 molar sodium hydroxide. It is simply a matter of multiplying these 2 numbers together to calculate the number of moles of sodium hydroxide used, therefore we have 0.0350 multiplied by 0.5 = 0.0175 moles of sodium hydroxide.
(b) To calculate how many moles of acid were needed we need to look at the balanced chemical equation for this neutralisation reaction:

From this balanced equation we can see that one mole of sodium hydroxide would require 1 mole of hydrochloric acid to produce 1 mole of sodium chloride in aqueous solution and 1 mole of water. Therefore as we had already worked out previously that we have 0.0175 moles of sodium hydroxide, there we would need 0.0175 moles of hydrochloric acid to complete the neutralisation, this is the answer to part (b)
(c) Since the question gave us the volume of hydrochloric acid actually used, 0.0250 dm3 and we know that this contains 0.0175 moles of hydrochloric acid we can work out the molarity of the hydrochloric acid using the following expression:
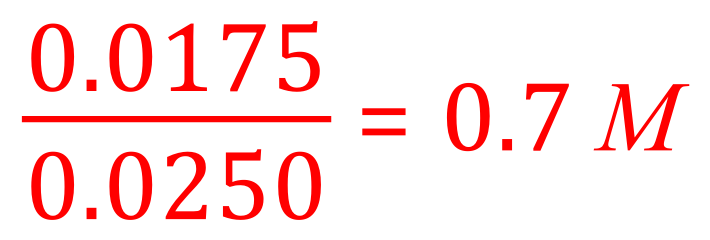
Q. During a titration experiment it was found that 37.6 cm³ of 0.5M (half molar) sulphuric acid was needed to neutralise 25 cm³ of potassium hydroxide solution.
(a) How many moles of sulphuric acid were needed to neutralise the potassium hydroxide?
(b) Given the equation for the reaction as below, calculate the concentration of potassium hydroxide solution used in the experiment.

A.
(a) We are told that the strength of the acid/concentration of the acid is half molar so we should be able to establish that this is 0.5 moles per decimetre cubed. We are told the 37.6 cm³ of this acid was used in this neutralisation therefore the number of moles would be:
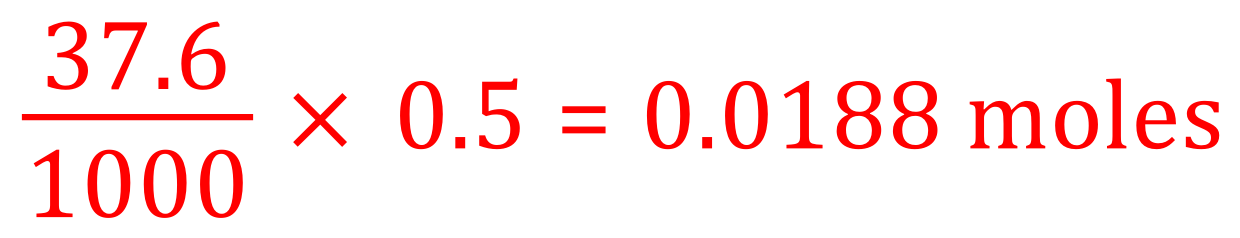
(b) From the balanced chemical equation we can see that one mole of aqueous sulphuric acid solution requires 2 moles of aqueous potassium hydroxide solution to produce 1 mole of aqueous potassium sulphate solution and 2 moles of water.We established in the previous part of the question that we did use 0.0188 moles of sulphuric acid therefore we are going to need twice as many moles of the potassium hydroxide solution, in other words 0.0188 x 2 = 0.0376 moles.Now that we have worked out how many moles of potassium hydroxide we used we can refer back to the information in the question which tells us that this was contained in 25 cm³ of aqueous potassium hydroxide solution. The molarity (moles per cubic decimetre) of potassium hydroxide is therefore given by the expression:
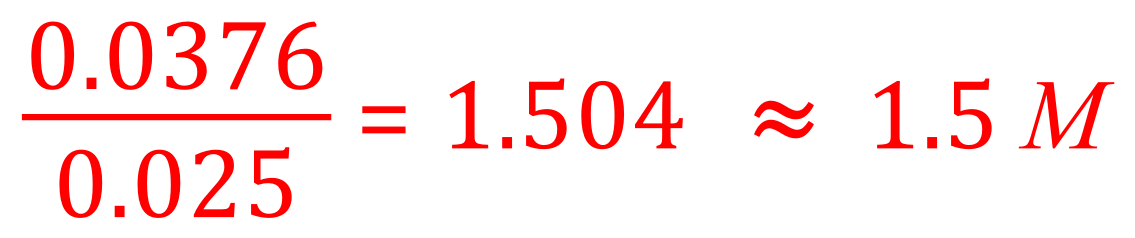
An alternative way to produce as a result would have been to use the improper fraction method:
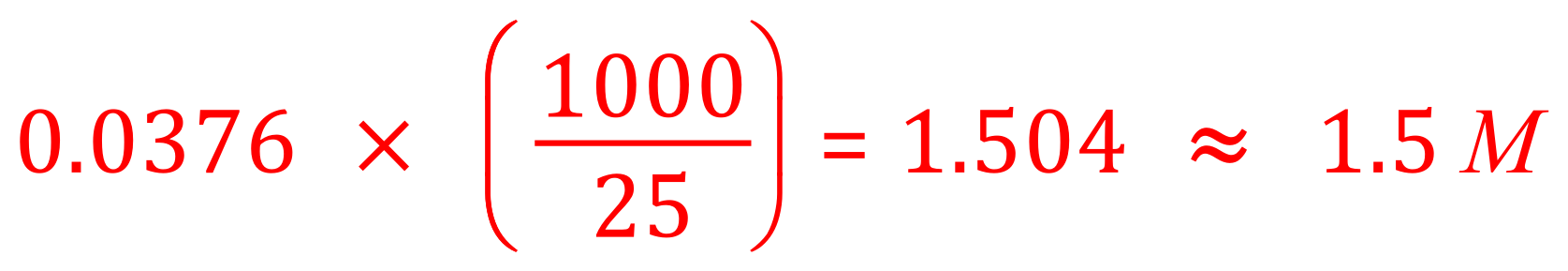
It is a personal preference as to which method you use, my personal preference is to use improper fractions because I know that I have 0.0376 moles in 25 cm³ and there are 40 "lots" of 25 cm³ in a cubic decimetre, so we simply multiply 0.0376 x 40 (that is 1000÷25).
>> Questions <<