Mass of Solute in Solution
Working out concentrations in moles or grams per decimetre cubed is relatively straightforward, however you may be asked in an examination question to work out the mass of a solute in a given volume of solution at a stated concentration. This is basically an exercise in manipulating data and isn't particularly difficult. We will now go through some examples to make sure that you understand what you're being asked to do.
Q. What is the mass of copper sulphate in 15 cm³ of a 2 molar solution of copper sulphate? The relative atomic masses you need are Cu = 63.5, S= 32, O = 16
A. You will always be given all of the information you need, so if you have a piece of information you don't use, then rethink what you're doing because you have probably done something wrong (having said that, sometimes "red herrings" can be thrown in although this is hopefully quite rare). Start by working out the relative formula mass/relative molecular mass of copper sulphate:

Having worked this out, we can now say that that would be 159.5 g of copper sulphate in a 1 molar solution, therefore in a 2 molar solution we would double this and so the grams per cubic decimetre contained in a 2 molar solution of copper sulphate would be 159.5 x 2 = 319g. We haven't answered the question now because we are asked to calculate the grams of copper sulphate in a given amount, 15 cm³ so it is a simple mathematical procedure to work this out. If there are 319 g in 1000 cm³ (1 dm cubed) then there would be:
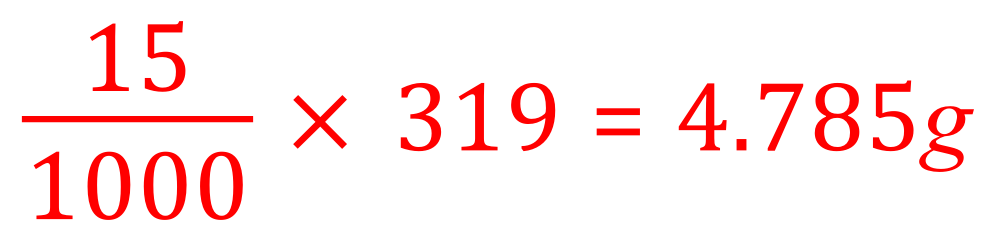
An alternative calculation to the one above would have been to work out first of all how many decimetres cubed we are dealing with and then multiply that by the number of grams in 2 moles. This would have been a simple task to convert 15 cm³ into decimetres cubed which would have been 0.015 decimetres cubed and then to multiply this by 319, we would have arrived at the same answer of 4.785 g. Whichever method you use you should ensure that you show all of your working because credit will often be given for incorrect answers provided that the working has been given and the examiner can see at which point you went wrong.
More often than not you will not deal with molarity, you will probably be given concentrations in "mass per volume", in other words "so many grams" in "so many cubic decimetres" of solution. With this in mind, let's take a look at a few more examples:
Q. Calculate the mass of sodium chloride in 555 cm³ of a 28.6 g per cubic decimetre concentrated solution.
A. In these sorts of questions we do not need to refer to relative atomic and relative molecular masses, these are simply exercises in basic arithmetic. We are told that we have 28.6 g in one cubic decimetre which we now know is equal to 1000 cm³, and we are asked to work out how many grams of sodium chloride would be present in 555 cm³ of this solution. This is a very simple fraction:

Q. Calculate the mass of solute in 80 cm³ of a 200 g per decimetre cubed solution of sulphuric acid?
A This is a simple mathematical permutation, we are told that we have 200 g of sulphuric acid and we know that one decimetre cubed is 1000 cm³. We are asked to calculate how much sulphuric acid would be in 80 cm³, and this is simply a fraction calculation:

It may be that you wish to calculate the given volume (80 cm³) in terms of cubic decimetres before you make the multiplication, in which case you would end up with a calculation of:
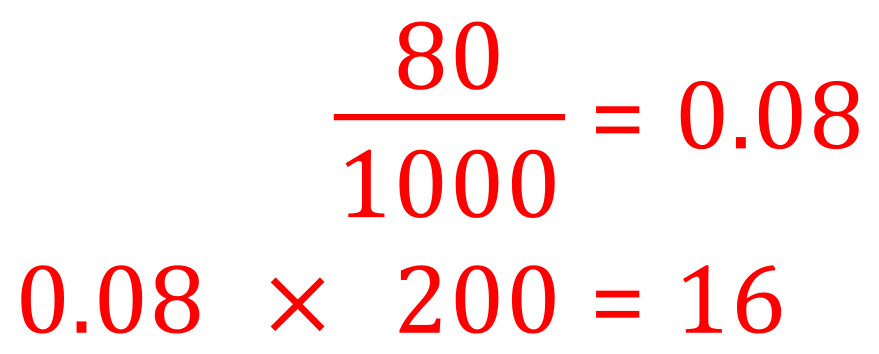
Q. Calculate the mass of potassium manganate (VII) in 39 cm³ of a 0.5 molar solution. The relative atomic masses are K = 39, Mn = 55 and O = 16
A. If you don't already know it, the best thing to do to start this sort of question is to find out the chemical formula for potassium manganate (VII) which you may also see referred to as potassium permanganate. Potassium permanganate has the formula of KMnO4 and Therefore a relative formula mass of 39+55+(16×4) = 158. From our study of moles and mole quantities you should remember that a 1 molar solution would therefore contain 158 g but we are talking about a half molar solution which would therefore contain half of a 158 which is 79. 39 cm³ in terms of decimetres is 39÷1000 = 0.039 dm3
The mass of potassium permanganate in 0.039 cubic decimetres of a solution of this concentration would therefore be:
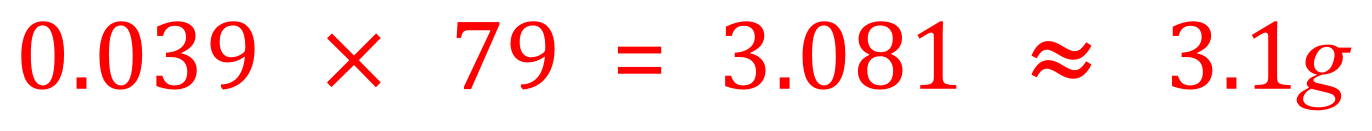
Q. What is the concentration in mol / dm3 of a solution containing 0.26 mol of a salt in 0.18 dm3
A. There is no mention of which salt, so clearly no Mr calculations. We are simply looking at proportions and fractions here. The question tells us that we have 0.26 mol in 0.18dm3
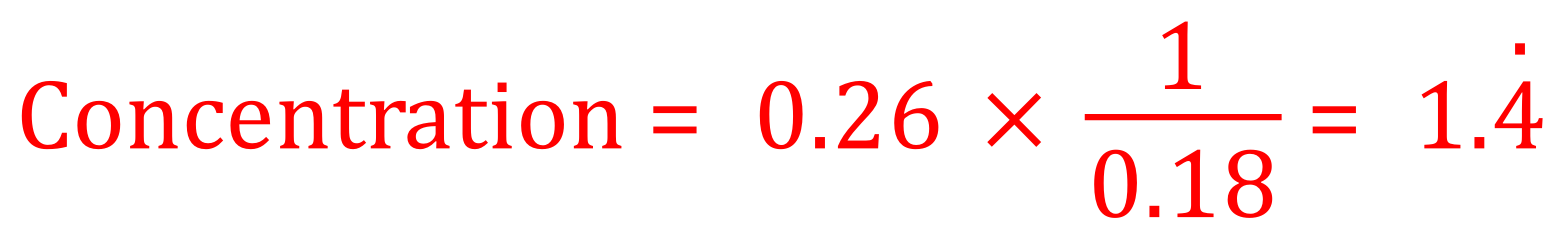
So if we have 0.26 moles of any salt in 0.18 dm cubed of solute then it is simply a matter of dividing the number of moles by the volume we have been given. It could have been that we had been given 0.26 moles in 2 dm cubed in which case the concentration would have simply been 0.13 moles per decimetre cubed, i.e. we would divide by 2. The dot above the number 4 shows that the decimal point value is recurrent.