[A] Basic Concentration Calculations In Solution
Q. A solution of sodium hydroxide has a concentration of 10 g per cubic decimetre, what is its concentration in moles per cubic decimetre or "molarity"?
H = 1, Na = 23, O = 16
We know that a mole of the substance contains the relative molecular mass in grams of the substance concerned, so what we 1st of all need to do is work out the relative molecular mass of sodium hydroxide. Given the relative atomic masses we can see that the relative molecular mass of sodium hydroxide would be 23+1+16 = 40
We are told that we have 10 g, not 40 g so the concentration in moles per cubic decimetre (moles per litre, or molarity) is:
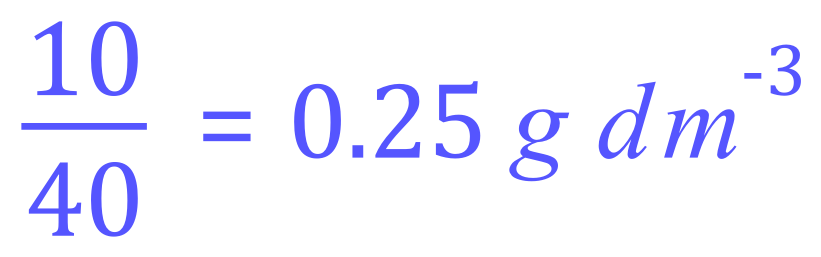
This would also be regarded as 0.25 molar, you would usually see this referred to as 0.25 M although it appears that grams per decimetre cubed appears to be the preferred method of showing units in current syllabi.
Q. What is the concentration in grams per cubic decimetre of a 1.25 molar solution of sodium carbonate?
Na = 23, C = 12, O = 16
The first thing we need to do, if we don't already know it is to write out the structure, symbolically, of sodium carbonate. You may be given this, you may not, I think it is more than likely that you would be.
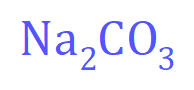
From this formula we can work out that the relative molecular mass is (23×2)+(12)+(3x16) = 106 so we can say that one mole of sodium carbonate would contain 106 g. It is simply a matter of multiplying this number by 1.25 to arrive at the concentration in grams per cubic decimetre of our substance:
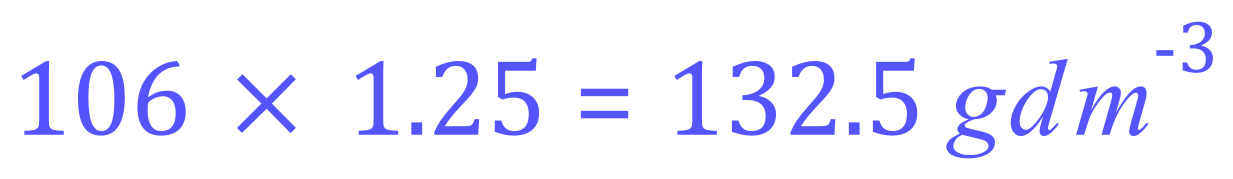
Q. A sample of sulphuric acid is found to have a concentration of 2.45 g per cubic decimetre, what is its concentration in moles per cubic decimetre?
H = 1, S = 32, O = 16
The beauty of this type of question is the fact that you are given the relative atomic masses, so it is an expectation that you will need to do something with them. We need to evaluate the relative molecular mass of sulphuric acid and as the question does not give you the formula for it you're expected to know this (this is unlikely to be the case in reality, but if you're studying at A level and you can't bring to mind the formula for sulphuric acid, I think you're in trouble :-))
The formula for Sulphuric Acid is of course H2S04 with a relative molecular mass of 98 (you can work this out for yourself if you wish) and we are told that the sample is found to contain 2.45 g of Sulphuric Acid per cubic decimetre:
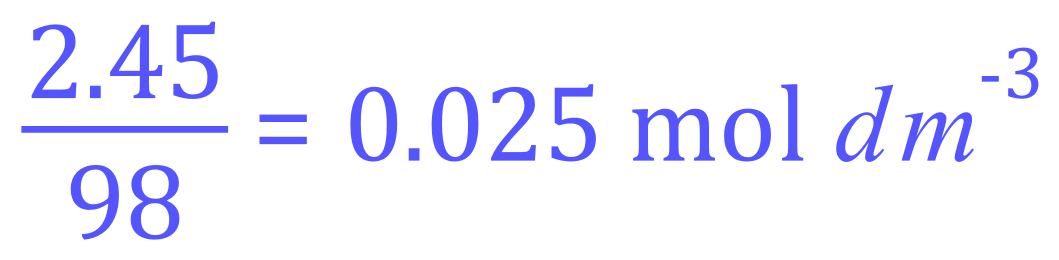
Let's take the last question little bit further. One of the things you will also require to do is to calculate the concentration of a substance (usually acid or base) by titrating it against a known concentration of another substance (the opposing acid or base until the neutralisation / endpoint is reached).
A student is given a sample of the above Sulphuric Acid, and conducts 3 titrations using 25 cm³ of Sulphuric Acid each time, titrating it against a solution of Sodium Hydroxide with an unknown concentration. The average of the 3 titrations is 12.5 cm³. From this information calculate the concentration in moles per litre of the Sodium Hydroxide solution.
In calculations involving "titrations" or as it used to be called "volumetric analysis", the balanced equation for the reaction needs to be derived:

Once you've got the balanced equation, things start to look a little clearer. We can see that one mole of Sulphuric Acid requires 2 moles of Sodium Hydroxide to produce one mole of Sodium Sulphate and 2 moles of water. Therefore the reaction is 1 to 2 with respect to the acid/base.
We know that the strength/concentration of the Sulphuric Acid is 0.025 moles per cubic decimetre, that is 0.025 molar so we need to establish exactly how many moles of Sulphuric Acid we have in our 25 cm³ sample:
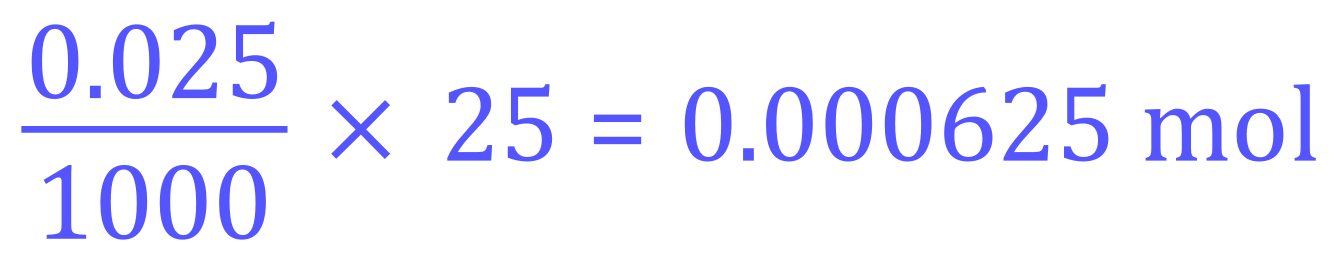
So now we can say that each 25 cm³ sample contains 0.000625 moles of Sulphuric Acid, and by virtue of the balanced equation we can conclude that we require twice as much (in moles) of Sodium Hydroxide for the neutralisation reaction, therefore we need (0.00065×2) equals 0.00125 moles, which we know is contained in 12.5 cm³ of the Sodium Hydroxide solution that we used:
Therefore the concentration of the sodium hydroxide solution used, in moles per litre would be:
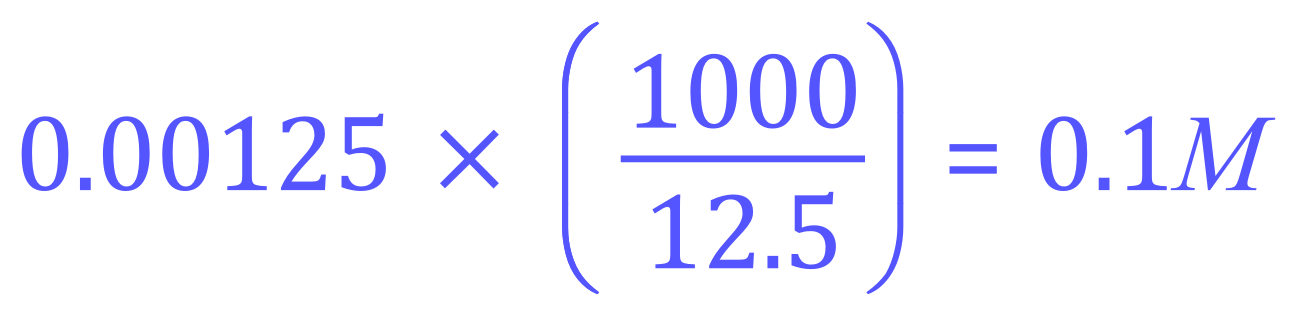
Back To >> Questions <<
Back To >> Calculations Regarding Concentrations of Solutions <<
Go To >> Solubility of Ionic Compounds << Chart
Back To >> Acids and Alkalis <<