[A] Naming Alkanes
If we consider the simplest alkane, which has only one carbon atom, we cannot actually draw this as a skeletal formula because it would only be a "dot", but because it only has one carbon atom it will be prefixed "Meth" and suffixed "ane", the first and simplest hydrocarbon is therefore Methane.
If you follow the table (at least across the first row) you will find that the next four Alkanes are "Ethane" "Propane" "Butane" and "Pentane" using the same rules as above.
|
1 |
2 |
3 |
4 |
5 |
|
Meth |
Eth |
Prop |
But |
Pent |
|
6 |
7 |
8 |
9 |
10 |
|
Hex |
Hept |
Oct |
Non |
Dec |
If Alkanes were this simple, we could now move onto the next family of hydrocarbons, however there is more to deal with yet, "substituents" and "branching" which occur in Alkanes to make them slightly more complicated.
Methane, and Ethane are the simplest of the Alkanes, so the naming of these is not going to cause much of a problem (hopefully) let's take a look at a slightly more complicated example, the next member of the family "Propane". Propane has a molecular formula of C3H8 and its skeletal formula looks like this:
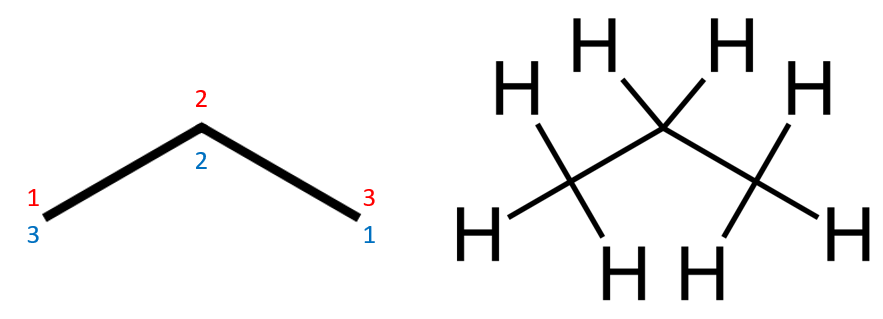
There are two things to remember here, first of all for the sake of simplicity, the skeletal formula does not show the hydrogens attached to the carbon atoms, the diagram on the left (with numbers, more on this shortly) is propane as is the diagram on the right. You need to remember, for the purposes of substitution that in Alkanes any "unsaturated" carbon will have a hydrogen atom attached to it. Any other substituent will be shown, hydrogen generally is not. The numbering, as shown is something that we have to do for when we start to decide on the naming convention. Depending on where substituent groups, branches et cetera are located. Also in the case of Alkenes and Alkynes, the numbering is quite important when judging the location of the double or triple bond.
The first rule in the naming of Alkanes (and this rule will be extended to the naming of other molecules subsequently, but we will concentrate on Alkanes for now) is to identify the longest carbon chain in the molecule and number it accordingly. In the case of propane of course there is only one chain which we number 123 (and in some texts you are advised to number it backwards as well, in this case 321 because it can help when you're trying to sort out your compound name, the substituents on a branched or substituted alkane have to keep with the lowest possible numbering system, you will see more shortly.
Let's try to make some sense of this, we'll take a look at a simple "branched" alkane:
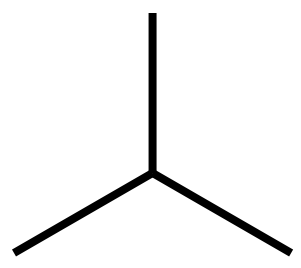
This is an example of a branched hydrocarbon, remember that each peripheral carbon atom will house three hydrogen is and the central one will have room for one hydrogen as three of its four possible bonds are taken up with bonding to the other carbons. Let us number the longest chain, you can see that whichever way you look at this molecule, the longest chain will be three carbon atoms, so we will select just for the sake of simplicity the lower ones.
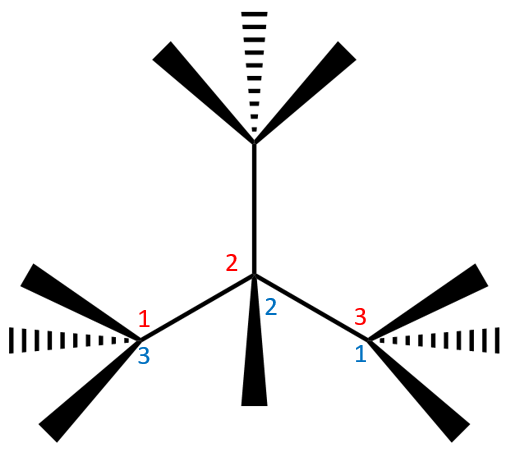
The longest carbon chain is three so of course we are dealing with a type of propane, but there is a substituent group coming off the second carbon atom. If you study the constituent group you should be at work out that it is a tetrahedral structure CH3 which appears to be a methane molecule CH4 with a hydrogen missing, and this is in fact what it is. If we call CH4 methane then you will understand that the naming convention calls CH3 "methyl".
|
1 |
2 |
3 |
4 |
5 |
|
Methyl |
Ethyl |
Propyl |
Butyl |
Pentyl |
|
6 |
7 |
8 |
9 |
10 |
|
Hexyl |
Heptyl |
Octyl |
Nonyl |
Decyl |
We have a methyl group joined to the second carbon atom of a propane backbone, we therefore call this molecule:

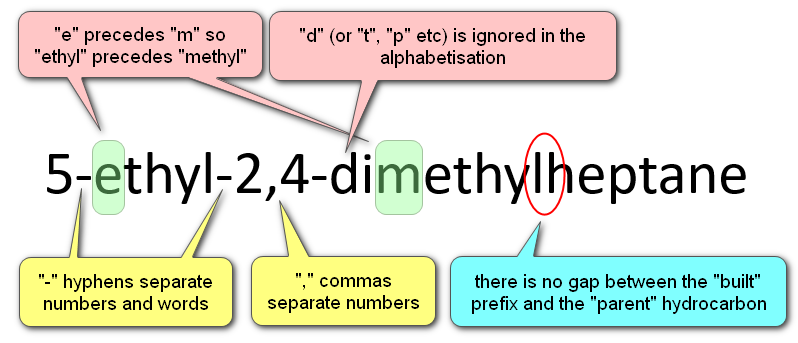
There are further rules and regulations to take into account as the name of the molecule gets more complicated, the hyphen between the "2" and "methyl" cannot be left out, and where more substituents and more numbering come into play, you will find that the numbers must always be separated with commas and the substituent groups and suffix (the parent hydrocarbon) will be separated with hyphens.
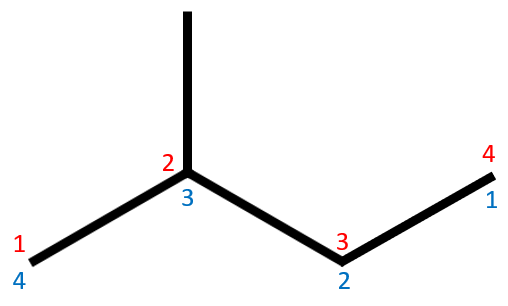
We will now come to a little twist in our naming convention. The molecule above is similar to the previous one but has one extra link in its chain. The numbering system shows us that there are four carbon atoms so we are dealing with a butane derivative. This is where the two sets of numbering come into the discussion. We know that the substituent group is a methyl group from our previous example but does it belong to carbon atom number three or carbon atom number two?
The rules state that substituents will be positioned so that they take on the lowest number, in this case it will be "2" which makes our branched hydrocarbon:

Notice that there is no gap between the substituent group name and the suffix (parent) alkane.
Lets now look at a longer example, but this time there will be two (or more) substituent groups on the main "carbon backbone" which will throw a new rule into the mix....the order of hierarchy in naming substituent groups.
Here is our substituted alkane:
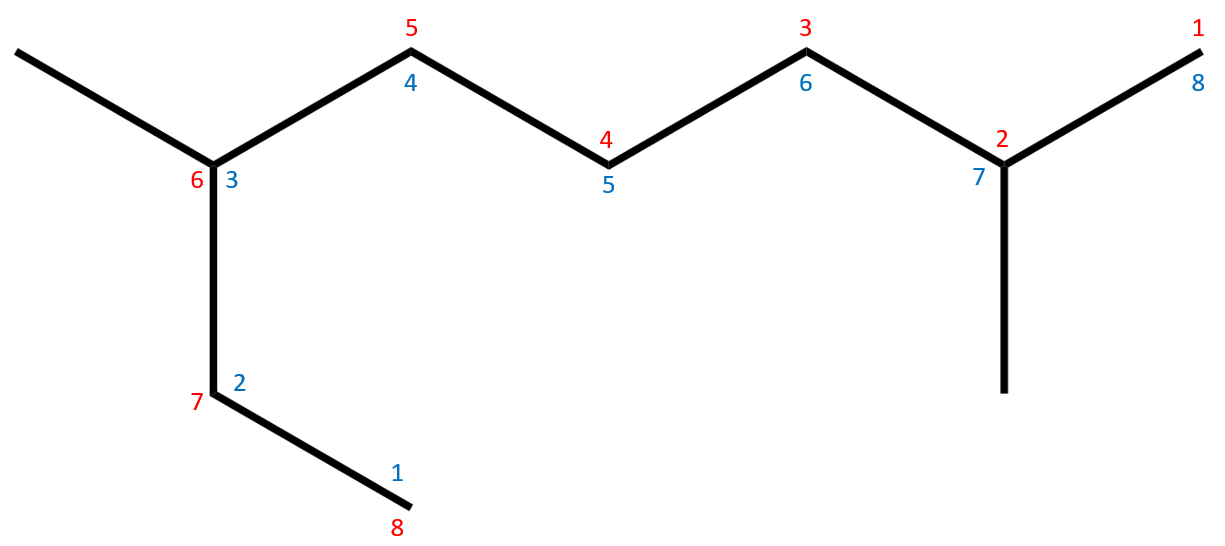
Identification of the longest chain is a little harder here, as you might default to looking along the horizontal and settle for 7, when in fact the longest is a choice of two 8 chain backbones. Now that we have identified a substituted Octane we have to look at the substituents BEFORE we decide on a numbering (red or blue). This is because we try to give the substituents the lowest number possible, so any substituent with a lower starting position will set the numbering. In this case we will number in red from right to left.
The substituents are BOTH ALKYL groups, hanging off carbon atoms 2 and 6, they have only one terminating carbon atom so are BOTH METHYL groups. We now need to name the molecule.
Starting from the substituent on the lowest numbered carbon atom, we have Methyl at position 2 and at position 6, our hydrocarbon is therefore a "di" methyl (having more than one of the same group on the molecule) and this is a combined prefix of number and name "di+methyl" = "dimethyl".
Finally we arrive at:

Now lets see an even tougher example:
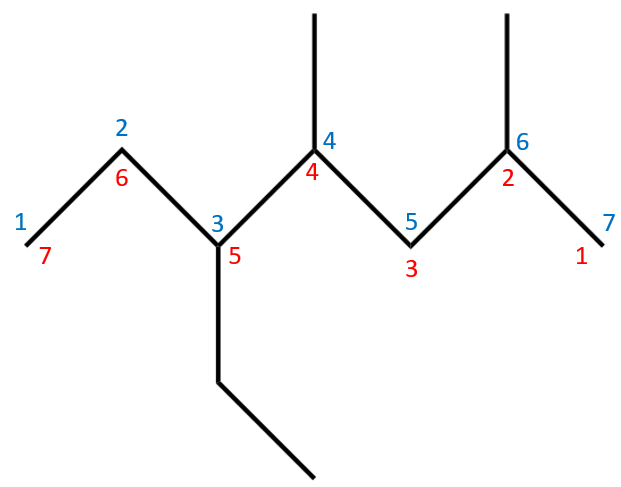
We apply the rules in this case in just the same way as in previous cases. First of all we identify the longest carbon chain, in this case we have a choice of two (have a look at this and you'll see why) I have chosen the horizontal chain and numbered it accordingly. Looking at our chain, you should be able to see the we have three substituent groups coming off it. We have an "Ethyl" group and two "Methyl" groups. We now have to decide which way to order them to correctly name the hydrocarbon.
We number the hydrocarbon so that the first substituent group we come to belongs to the lowest number carbon atom can possibly have. If you were to start from the left using the blue numbering system, the first substituent group would come at carbon atom number three, but if we use the red numbering system, first substituent group comes carbon at number two. So this is the numbering system we use (red).
We now have to decide the order of precedence for the substituent groups. The rule is quite simple here, they are dealt with alphabetically, so as "Ethyl" comes before "Methyl" this is the order in which the substituent groups will be numbered and subsequently named in the molecule. We therefore count along our red numbering system and find that our Ethyl group appears at carbon atom 5, and our methyl groups appear at carbon atoms 2 and 4.
Before we actually make an attempt at a name, we should note the fact that we have two of the same type of group as substituents. We have two methyl groups so we need to prefix methyl with its Latin prefix "di" denoting two, and combine this as "dimethyl".
This does not affect the alphabet system, the the fact that dimethyl precedes ethyl alphabetically makes NO difference, the dimethyl is still regarded as methyl for the purposes of the naming convention.
Now we can look at our molecule and give it a name:

Let us break this name down into its component parts and identify what all of these hyphens and commas as are there for.