[A] Naming Halogenoalkanes
Hydrocarbons do not have to only be substituted with alkyl groups, there are other "functional" groups which can be attached and these will affect the nature properties and nomenclature of the substance that is produced. If we take a simple Alkane, for example Propane, and replace one if the terminal hydrogen atoms with, say, Bromine:
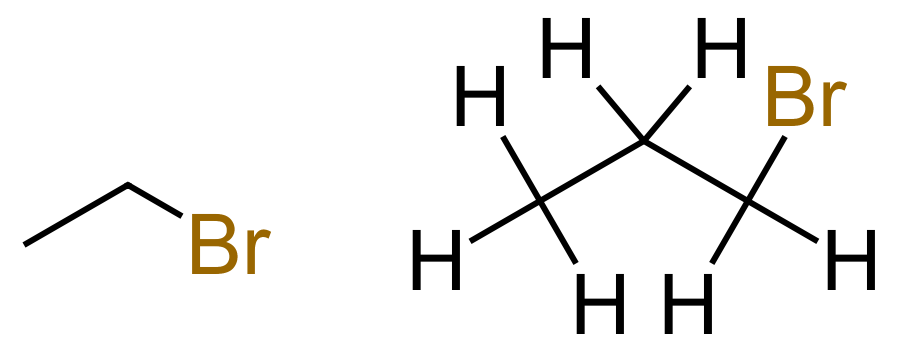
We still have an Alkane, but now it is a halogeno-alkane, in this particular case it is Bromopropane. We could have called this 1-Bromopropane but conventionally when the substituents are at the end of the molecule in the number one position, always there is no ambiguity about numbering we often leave out the prefix number. When we move into longer chain alkanes, the numbering will become more important, more often than not compulsory.
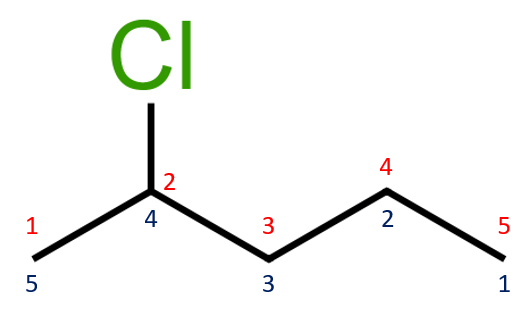
This time we choose a longer chain alkane and have substituted it with a single chlorine atom. The numbering convention that we've used previously is displayed and just the same way as we did with Alkyl groups , we name the hydrocarbon (or in this case halogenoalkane) so as to give the substituent the lowest number. We therefore have a molecule of 2-chloropentane.
If we have more substituent groups, we adopt the alphabetical system, therefore chloro- would be preceded by any bromo- which happened to be there, followed by fluoro-then iodo-. Any substituent alkyl groups would be inserted according to their position in the alphabet so for example any ethyl groups would come after chloro- but before fluoro-
What would we call this molecule?
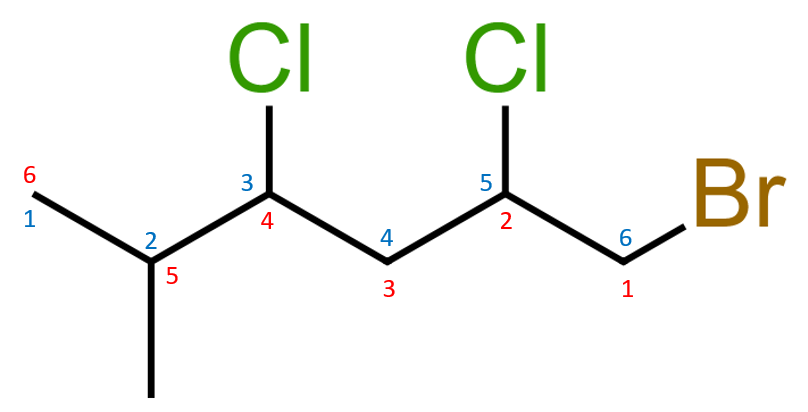
Naming this substance is no different from any of the others you've seen so far. A number of the preliminary steps have already been done, namely the identification of the longest chain which in this case is six carbon atoms long, so we are ultimately looking at a substituted hexane.
We start the numbering so that the first component encountered has the lowest number, this therefore means that we use the red system because we have a bromine atom substituted at position one. Moving from right to left we have chlorine atoms at positions two and four and a methyl group at position five.
We can therefore name our molecule accordingly:
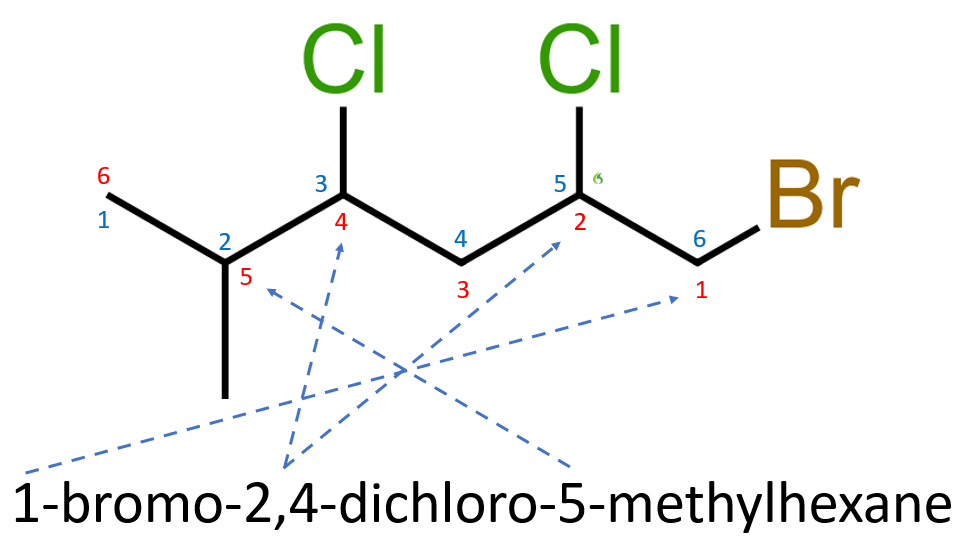
Remembering once again with that in the naming convention, we remain in lowercase with hyphens between numbers and letters, commas between numbers and no spacing between the last mentioned subsidiary group and the parent hydrocarbon name.
Before we leave this, I'm going to look at one more quite substantial molecule and just name it, I would like you to study it and see for yourself where the name came from. Follow the rules about nomenclature and you should be able to reproduce the name.
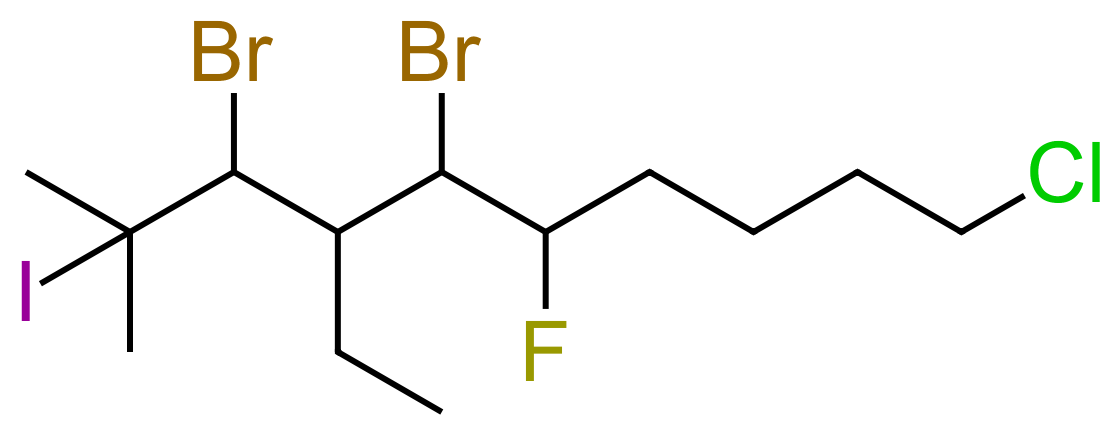
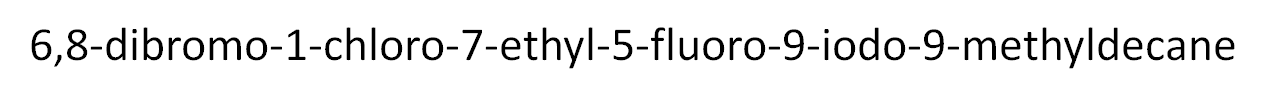
Unfortunately, it gets harder than this, we haven't looked at ring structures, carboxyl groups, multiple unsaturations etc yet!!