[A] Naming Alcohols
We saw previously that Alkanes can be substituted with alkyl groups, halogens and looked into the naming some of the compounds which resulted from this. Certain substituent groups will change the base name of the hydrocarbon, resulting in the changing from one specific type of organic molecule to another i.e. it becomes a member of a different family. One such family which we will look at now, are the alcohols, the simplest of which are straight-chain Alkanes (or Alkenes, or even Alkynes) with one or more hydroxyl groups attached.
The simplest alcohol is Methanol:
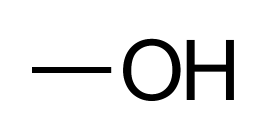
The skeletal formula isn't brilliant, it looks like there a hydroxyl group hanging in mid air with nothing attached to the left, however you have to remember that the "end of the line" at the left is a terminating carbon atom complete with three hydrogen atoms, so the molecule is really:
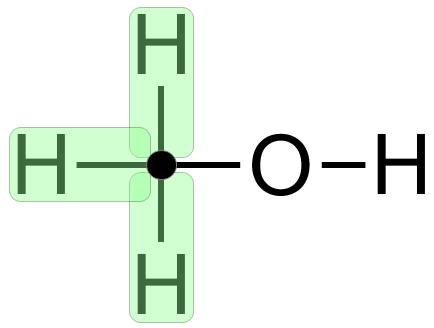
Using very short chain hydrocarbons / carbon backbones doesn't make the explanations very clear, so I'll avoid them from now unless its really necessary to go simple again.
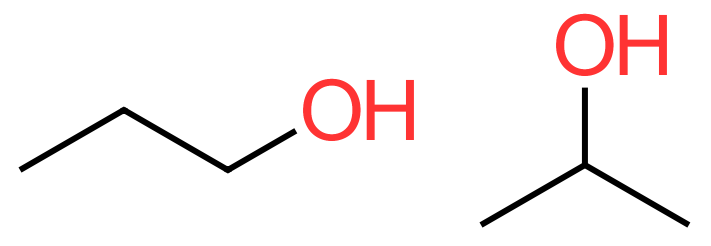
The two molecules above are both alcohols derived from propane. The leftmost molecule shows the "hydroxyl group" or "alcohol group" attached to the terminal carbon which, following our numbering convention would be number one. The molecule to the right has the same molecular formula (the same number of carbon, hydrogen and oxygen atoms in total) but the structure is slightly different, as the alcohol group is now attached to the middle, or carbon number two. These alcohols display what is known as isomerism and are known respectively as:
1-propanol / propan-1-ol and 2-propanol / propan-2-ol
The first isomer "1-propanol" is often just referred to as propanol but to be specific about its isomer we have to make sure that we include the number 2. It is the position of the hydroxyl group on the carbon backbone which dictates the numbering sequence, as we are no longer deal with an Alkane, we now have an Alcohol. The IUPAC convention seems to favour the splitting of the base name to insert the number which indicates the position of the hydroxyl group on the carbon skeleton, so we would preferably say propan-2-ol rather than 2-propanol.
Perhaps the most important thing in the naming of these compounds is that we have to remember that the hydroxyl group outranks everything, so for example a substituted alkane with a hydroxyl group and a halogen would be a "halogeno-alcohol" and not a"hydroxy-haloalkane". Perhaps one final example here will clarify this:
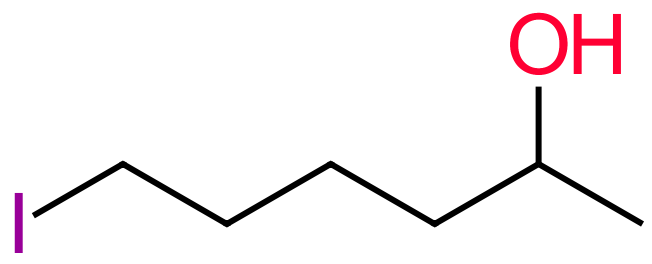
Following the rules and identifying the longest chain, we have to take into account the fact that the longest chain has to contain the hydroxyl group. Remember that the iodine atom is a substituent so we are looking at a six carbon chain or a derivative of hexane.
