[A] Naming Alkenes and Alkynes
The presence of a double or triple bond generally alters the name of the hydrocarbon to an alkene or alkyne derivative. If we take a look at our simplest alkene and our simplest alkyne to start with this may start to make things a little bit clearer:
Not the most exciting pictures you're ever going to see in your studies of organic chemistry, but nonetheless, two quite important substances. The molecule on the left is a molecule of Ethene (formerly known as Ethylene) and Ethyne (formerly known as Acetylene).
The red circles and blue circles have been put there to remind you that the terminations are in fact carbon atoms, in the case of the first molecule there will be two hydrogen atoms attached to each terminating carbon and in the case of the right-hand molecule there will be only one on each end, however they are there nonetheless.
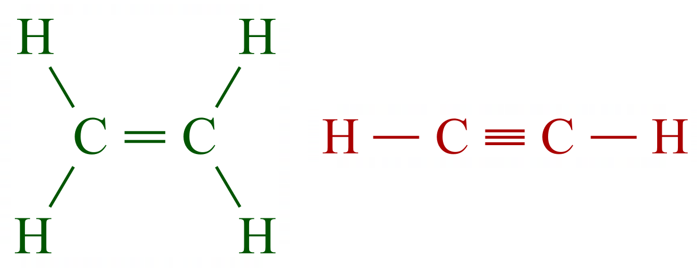
The two molecules here are replicated in their "displayed formulae", which probably makes more sense for smaller molecules, as we have mentioned previously, the skeletal structure is more ideally suited to larger molecules where the peripheral hydrogen atoms would overcrowd the drawing. I will show these to molecules once more in their electronic "view" the "dot and cross" structure so you can get a picture of the electronic involvement in the bonds (although that is more complicated than it would appear, more on that later).
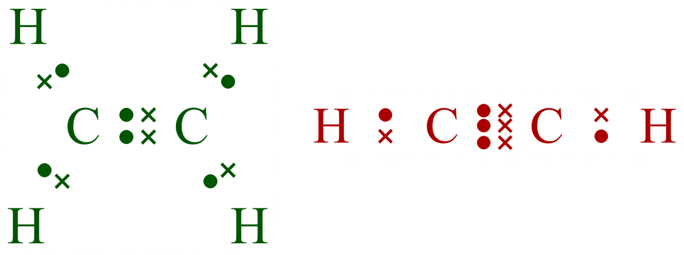
As you've probably already worked out, the dot and cross structures are far more informative than the skeletal, but would very quickly overwhelm any diagram you were trying to draw.
We have seen so far, that certain functional groups will alter the naming of the molecule because the "family" that the molecule comes from has changed. For example the addition of a hydroxyl group to alkanes gives us alcohols, and the addition of a halogen will give a "halogeno-alkane"
We have to make sure that we identify peripheral groups, as well as maintain the hierarchy. Unfortunately when we throw into the mix double and triple bonds it gets a little bit more complicated.
Let us take a look at the two isomers of Butene:
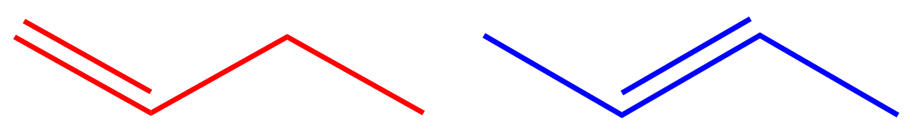
I chose Butene over Propene because in the latter case (Propene) there is only one place where the double bond can go, between carbons one and two (if you try to put the bond between carbons two and three, flip it over and you're back at one and two!).
We take the numbering system once again but this time we have to recognise the fact that there is a double bond and give it the lowest position in the carbon backbone.The red molecule has four carbons (as you would expect) with the double bond starting at carbon atom number one, therefore this molecule would be called 1-Butene, or But-1-ene to formally identify where the bond lies. In the case of the blue molecule, the double bond starts (irrespective in this case of which end you start) carbon atom number two, so this substance would be called 2-Butene or But-2-ene.
When we were looking previously at multiple instances of the same functional group, for example having two methyl groups, we handled this by prefixing the methyl with "di" to show that there were two of them. Had there have been three, we would have used "tri" and so on. We do the same thing with Alkenes (and subsequently you will see, Alkynes) where there are multiple instances of double and/or triple bonds:
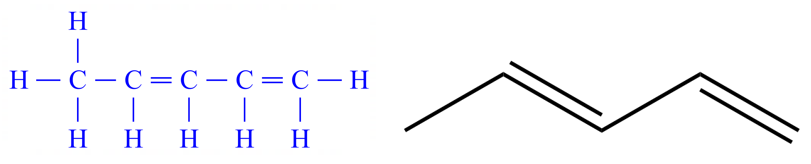
This diagram is a representation of a doubly unsaturated (that is two double/triple bonds) hydrocarbon. The longest carbon chain is five, so are looking at some form of Pentene. We have to adopt a numbering system which gives the double bonds the lowest number in the carbon chain, so you should be able to see that if we work from right to left we will have a double bonds starting at carbon atom number one and a further double bond starting a carbon atom number three. I mentioned previously that more than one unsaturation would require the use of a prefix "di" or "tri" and in this case we need to use the first one. We have in fact a "diene", we have identified the longest carbon chain as five and we now know where the two double bonds start respectively.
We can now name our hydrocarbon compound:

Sooner or later we come up against a problem where dissimilar substituent groups which would independently attempt to rename the "base" hydrocarbon appear in the same molecule. For example, we could have a hydroxyl group which would attempt to rename the base hydrocarbon to an alcohol, and a double bond/triple bond which would attempt to rename the (for example) alkane or alkane derivative to the corresponding alkene or alkyne:
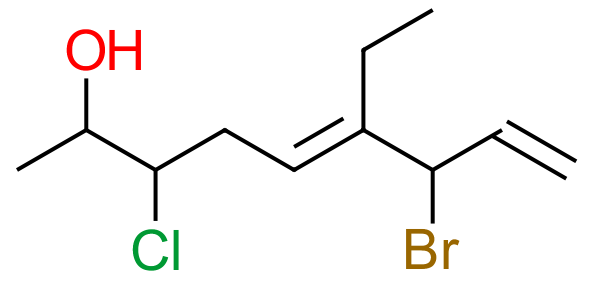
This somewhat worrying looking molecule is going to be moderately difficult to name, but there is no reason why we can't actually do it if we simply follow the rules and take it nice and steady, making sure that we don't miss out any of the steps on the way.
Step 1 - identify the "base" hydrocarbon by counting the number of carbons in the longest chain. When you do this you have to make sure you take into account the side chains just in case what might appear to be a side-chain is in fact going to make your molecule longer, you need to watch out for this. Taking into account the side-chain we still have a horizontal row of nine carbon atoms so we are dealing with a "nonane" derivative, of course this is going to change and because we have hydroxyl groups and double bonds to take into account it is not going to retain its alkane suffix for long!
Step 2 - we have seen in the hierarchy of naming substituent groups, that hydroxyl seems to (at the moment) outrank everything, so we are going to be working eventually with an alcohol, with a hydroxyl substituent at carbon atom number two.
Step 3 - In the hierarchy, double and triple bonds take next precedence, so we look to see if we have any of those and of course we have two. Remember that the double bonds are numbered at the point at which they start so we have a double bond at carbon atom number five and a double bond at carbon atom number eight. Two double bonds in the same molecule tells us that we are going to be dealing with ultimately a "diene", or more correctly a "dienol" (note that affixing the "di" removes the need for the extra "e").
Step 4 - We have two halogen substituents at carbon atoms three and seven, as well as an ethyl group at carbon atom six. Now that we have identified all of the component parts we string them together to form the name of the molecule.
Remembering the order of precedence:
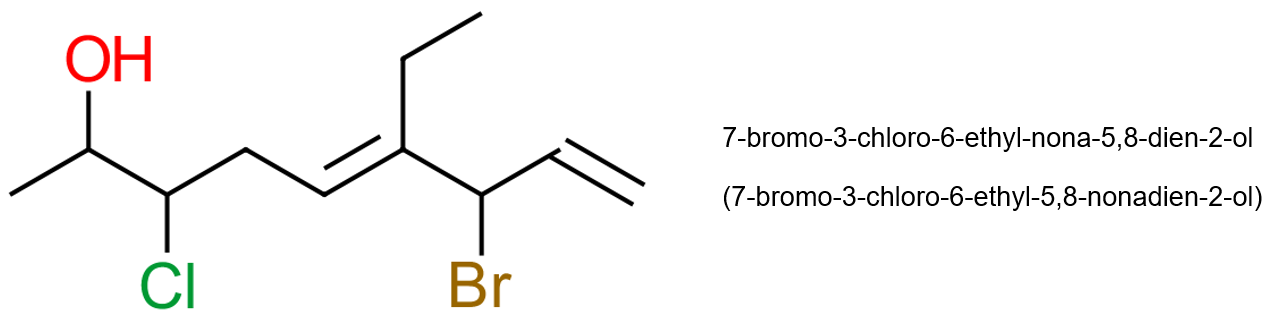
We treat triple bonds, and molecules where there are combinations of double and triple bonds, in just the same way. The steps are exactly the same. Let's take a look at a mixture of substituents in our next molecule, and attempt to give it a name:
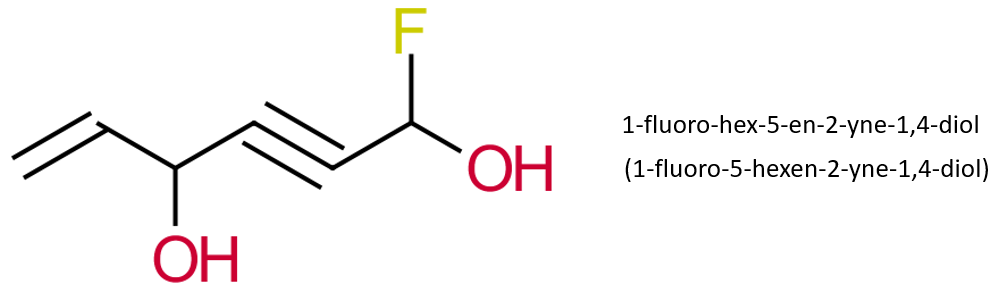
Work through the steps to see if you can replicate the name.