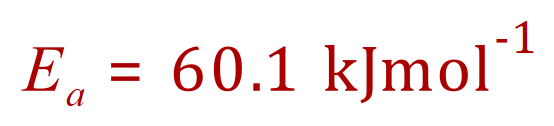[A] The Arrhenius Equation
This equation seems quite fearsome when you first encounter it, but there is a way in which the equation can be expressed which takes a little bit of the edge off it. We will come to that soon, but first of all let us take a look at this equation which links together things like rate constants, activation energies, gas constants and temperatures. It also explains why the rate of reaction will increase with increasing temperature:
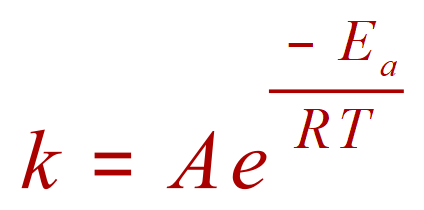
Where ‘k’ is the rate constant, -Ea is the activation energy of the reaction, R is the Gas Constant, T is the temperature and A is the Arrhenius Constant. the Arrhenius Constant is reaction specific, so it’s value will vary but of course R the universal gas constant will always be fixed at 8.31 Joules per kelvin per mole. As is the case with many equations, it is likely that you will be given all but one value and be asked then to manipulate the equation in terms of the unknown and obtain it. Let’s take a look at the example of this:
Q. Find the activation energy of a reaction given the values below:
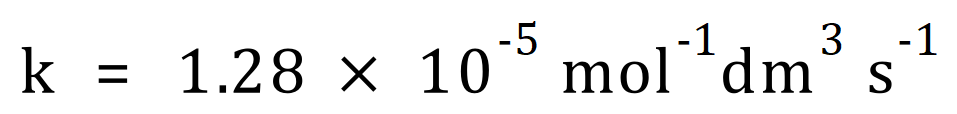
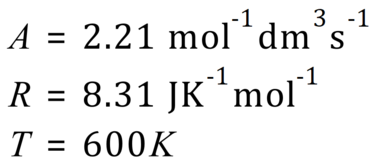
A. As expected, you are given all of the values you need except the one you need to calculate (obviously!). Your mathematical dexterity is now needed to convert the Arrhenius equation into a format in terms of the activation energy and once you’ve done that you will be able to plug in your values to obtain the required result.
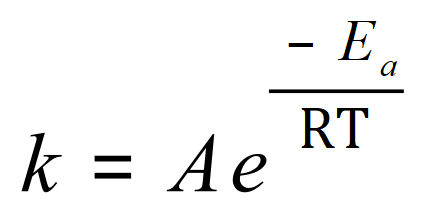
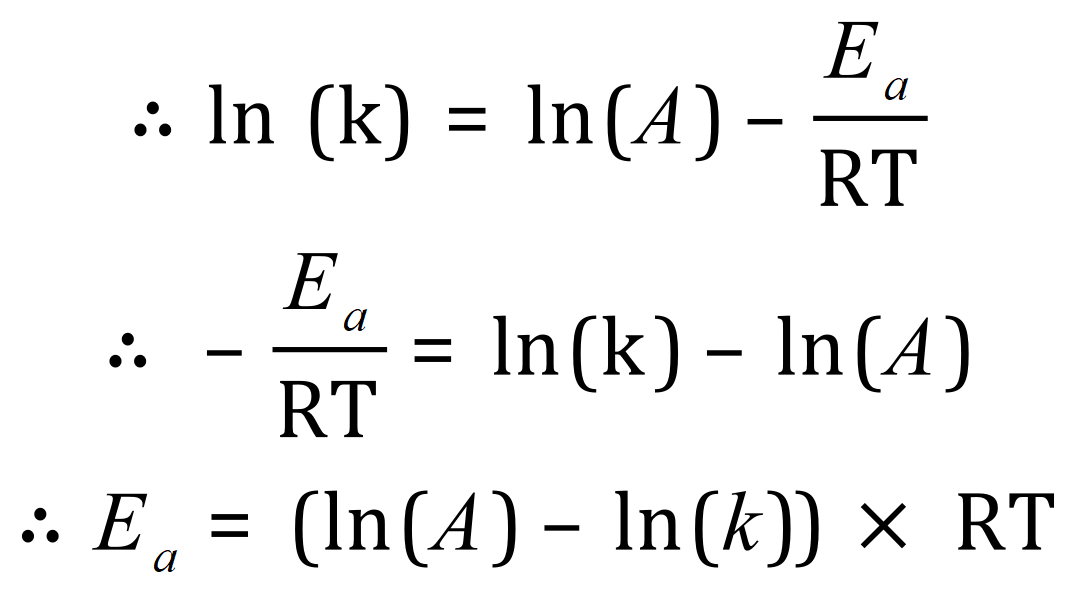
We can now "plug" in some values such as the natural logarithm of A, k and we have the values of R and T:

Therefore: