[A] The Rate Constant "k"
So what exactly is the “rate equation”?
Well, it is an equation which links the concentrations of the reactants and the effect of the individual components on the rate of the reaction, and is given by the expression shown below:
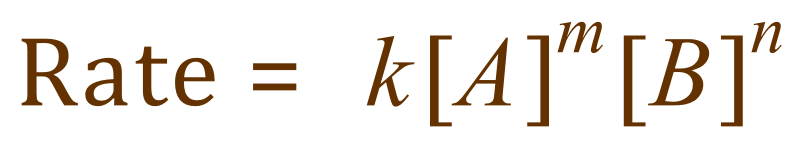
Where ‘k’ is an entity known as the rate constant, A is the concentration of reagent A, and B is the concentration of reagent B. The index notations m and n are what are known as the “orders of reaction” and these determine what effect changing the concentration of any of the reagents would have on the rate of the reaction.
If m or n equals zero, then the reaction rate is effectively independent of the concentration of the reactants, in other words if you increase or decrease the concentration of any of the reactants, this would have no effect on the overall rate.
If m or n equals one, then the reaction rate is proportional to the concentration of the reagents, in other words doubling the concentration of a particular reactant will double the rate, likewise tripling or quadrupling would have the same effect, also halving a concentration of a reagent with an order of one would halve the reaction rate accordingly.
If m or n equals two, doubling the concentration of that particular reagent will have the effect of increasing the rate of reaction by fourfold, similarly trebling the concentration would increase the rate of the reaction by ninefold, you can probably see that if the reaction order is 2 then doubling the concentration would be “2 squared”, similarly trebling the concentration would be “3 squared” and so on.
The overall rate of the reaction is the sum of the reaction orders m + n
It should be noted that the order of the reaction cannot be found by simply looking at the balanced equation, orders of reaction can only be found by experimentation.
Let us try to make some sense of this by looking at an example:
Q. The reaction between nitric oxide NO and carbon monoxide CO with oxygen yields nitrogen dioxide and carbon dioxide according to the balanced equation given. at a given temperature ‘T’ the rate of the reaction is as shown:

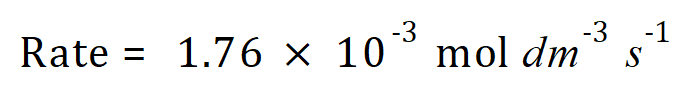
This rate is effective when the concentrations of the three component reagents are the same, that is:
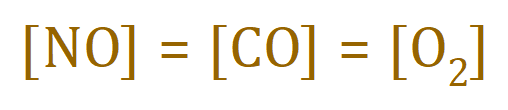
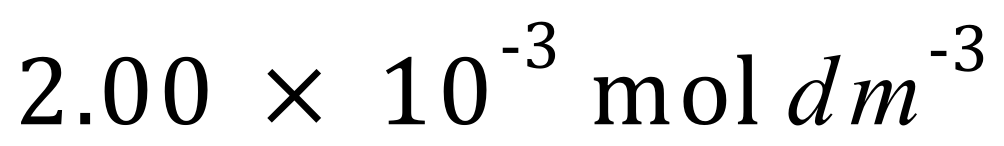
Given that the order of the reaction with respect to nitric oxide is 2, and the orders of the reaction with respect to carbon monoxide and oxygen are both zero, calculate the rate constant ‘k’.
A. First of all, we should write out the rate equation for this:
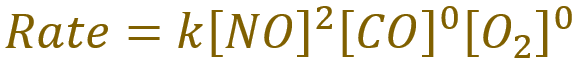
Because anything raised to the power of zero becomes one, so the concentrations of carbon monoxide and oxygen simply equate to one, the above rate equation simplifies to:
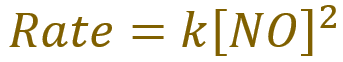
Rearranging the above equation in terms of ‘k’, which is the entity that the question has asked us to find, we arrive at the following expression:
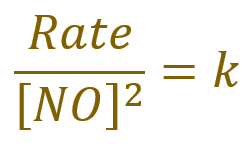
Which evaluates numerically to:
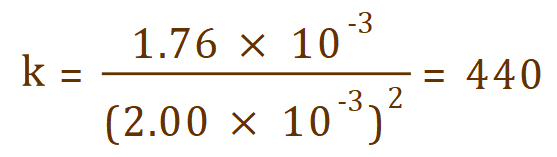
This is of course only half of the job. What we need to do now is evaluate the correct units for the rate constant, and this can be quite tricky. First of all let us look at the units that we have for reaction rate and concentration of nitric oxide (which, to complicate matters. … is squared).
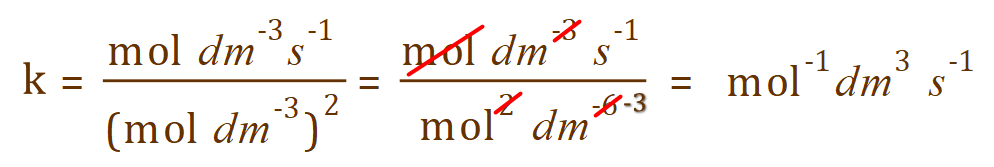
Having completed this, you should now quote your answer like this:
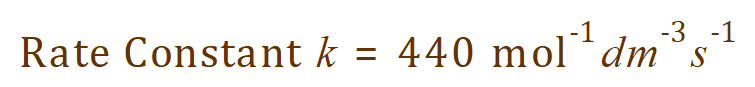
Now who was it who said that chemistry wasn’t fun?
You would think that an entity like a rate constant would have a fixed set of units, after all energy is always Joules, concentration is moles per litre, mass is kilograms and so on and so forth. The rate constant however has units which vary according to the overall order of the reaction. The above example concerned a reaction with an overall order of two, we are now going to take a look at another example where the overall order is three and we will notice a difference in the units of the rate constant ‘k’.
Q. This time we will take a look at the reaction between nitric oxide and chlorine, producing NOCl (Nitrosyl Chloride) in a reaction which becomes reversible above 100°C. The balanced equation for this reaction is as shown below:
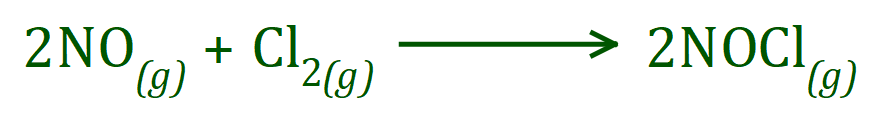
This reaction is second order with respect to nitric oxide and first order with respect to chlorine, the rate equation is therefore:
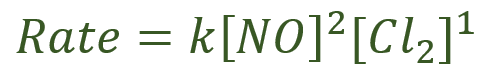
A. We are told that the rate of the reaction at 50°C is 5.85 x 10^6 moles per decimetre cubed per second when the concentrations of nitric oxide and chlorine are the same at 0.4 moles per decimetre cubed. In other words:

Our task this time is to calculate the rate constant, based on the information that we have. The expression for the rate constant then, rearranged and taking into account the information we’ve been given is as follows:
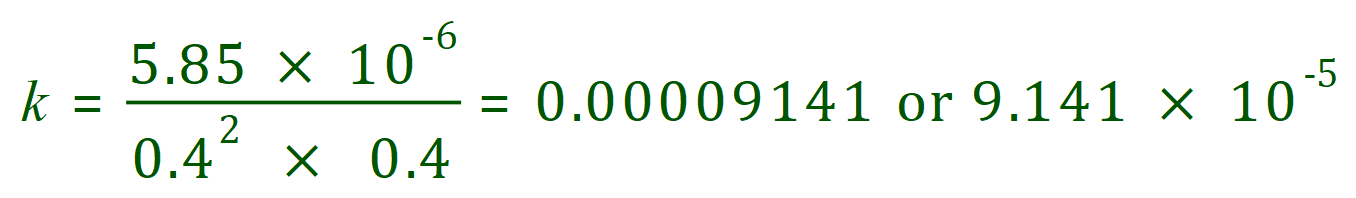
So you can see that the answer to the question is that the rate constant is in fact the number given, but if only things in life were that simple…… as we now have to calculate the correct units to express our answer. Using the above formula, but this time just manipulating the units we have the following:
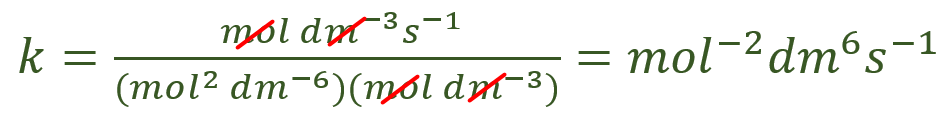
So we would present our answer to the question as follows:
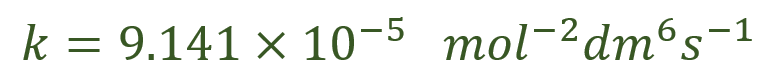
The question would probably ask you to quote your answer to a certain number of significant figures, or decimal places, but I’ve decided to leave the answer at its full calculator accuracy to emphasise the fact that no “rounding” should take place until you are at the end of the question.