Balanced Equations and Reacting Masses
If you know the masses of the reactants and products taking part in a chemical reaction, then from this information you can work out the balanced simple equation for the reaction.
1. Work out the number of moles of each substance by dividing the masses that you have by the respective relative molecular masses.
2. Looking at the number of moles you have of each substance, divide each number by the smallest number, so for example if you had 2 4 6 and 8 you divide each number by 2.
3. If any of the numbers in the calculation above are not integers, multiply up by a suitable multiplier to make that the case, so for example if your numbers were 1.5, 2.5, 4 and 5 you with multiply everything by 2 to make it 3, 5, 8 and 10
4. Write out the balanced symbolic equation for the reaction by putting these newly calculated numbers in front of the substances that they refer to.
This probably sounds quite confusing, let us take a look at a worked example to clarify things:
Q. 4.6 g of sodium reacts with 1.6 g of oxygen to form 6.2 g of sodium oxide. Using these reacting masses, write the balanced simple equation for the reaction. You are given the relative atomic masses of O = 16 and Na = 23. The chemical formula for sodium oxide is Na20 with a relative molecular mass of 62.
A. Follow the steps outlined above. This particular question is not ideal, because it does not state whether we are talking about oxygen atoms or oxygen molecules. We must assume that if the sodium is being burnt in air we are talking about oxygen molecules 02.
4.6 g of sodium divided by 23 which is the relative atomic mass gives us 0.2 moles
1.6 g of oxygen divided by 32 which is the relative molecular mass of an O2 molecule gives 0.05 moles
6.2 g of sodium oxide divided by 62 which is the relative molecular mass of sodium oxide gives 0.1 moles
If we divide each of these numbers by the lowest one which is of course 0.05, we arrive at a ratio of 4 sodium to 1 oxygen molecule to 2 sodium oxide, from this we can construct our balanced equation thus:

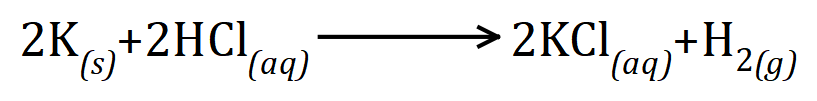
Q. 2.34 g of potassium is reacted with 2.19 g of hydrochloric acid to form 4.47 g of potassium chloride and 0.06 g of hydrogen. Given the relative atomic masses of hydrogen, chlorine and potassium as 1, 35.5 and 39 respectively use the reacting masses to produce the balanced chemical equation for the reaction.
A. As before, the first thing that we have to do is work out the number of moles of each substance involved.
2.34 g of potassium divided by 39 which is the relative atomic mass of potassium gives us 0.06 moles
2.19 g of hydrochloric acid (relative molecular mass 36.5) gives us 0.06 moles
4.47 g of potassium chloride (relative molecular mass 74.5) gives us 0.06 moles
0.06 g of hydrogen (relative atomic mass 1, but we use 2 as we are assuming diatomic hydrogen) will give us 0.03 moles of diatomic hydrogen gas.
If we divide each of these numbers by the lowest one, in this case 0.03 we arrive at a ratio of potassium (2), hydrochloric acid (2), potassium chloride (2) and hydrogen (1).
From this we can construct the balanced chemical equation thus:
Q. 1.2 g of a hydrocarbon Z combusts completely in 4.48 g of oxygen to produce 3.52 g of carbon dioxide and 2.16 g of water. Given the relative atomic and molecular masses of Z, 02, H2O and CO2 as 30, 32, 18 and 44 respectively write the balanced symbol equation for this reaction.
A. Do not let the fact that you don't know the identity of one of the components faze you at all, because you are given its relative molecular mass and really this is all you need.
1.2 g of the unknown hydrocarbon Z divided by 30 gives us 0.04 moles
4.48 g of oxygen divided by 32 (diatomic) gives us 0.14 moles
3.52 g of carbon dioxide (44) gives us 0.08 moles
2.16 g of water (18 gives us 0.12 moles
If we divide each of these numbers as we have done previously, by the lowest number which in this case is 0.04 we arrive at a ratio of 1 mole of Z, 3.5 moles of oxygen gas, 2 moles of carbon dioxide and 3 moles of water.
We have a non-whole number, so we multiply everything up by 2 to get rid of the non-whole number, therefore Z = 2, O2 = 7, CO2 = 4 and H2O = 6
From these values we can now construct the balanced symbolic equation for the reaction:
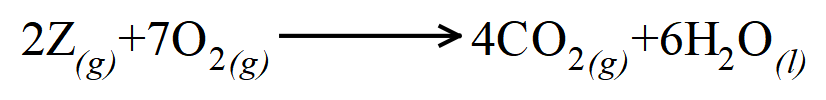
Technically that is the end of the question.
However for extra points we may be asked to suggest an identity for the hydrocarbon Z. Using the law of conservation of mass we can see that we end up with 4 carbon atoms at the end of the reaction therefore we must have started with 4. 2 units of hydrocarbon Z contains 4 carbon atoms so we can say that the carbon part of the hydrocarbon is C2 . Once again using the law of conservation of mass we can see that we end up with 12 hydrogen atoms (bonded into 6 water molecules) so we must have started with 12 hydrogen atoms. Again we can see that 2 units of Z would have held these 12 hydrogen atoms so we can safely conclude that the hydrogen part of Z would have been H6
A possible identity for hydrocarbon Z would therefore be C2H6 which is Ethane.