Electrolysis Of Molten Ionic Substances
Q. Predict the products of the electrolysis of molten Sodium Chloride. Write out the half equations occurring at each electrode and the overall reaction taking place.
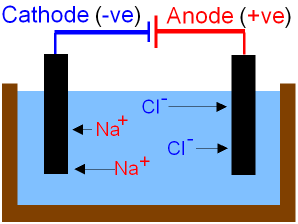
You can if you want to, draw a small diagram like the one shown but unless it is specifically asked for in the question, your time might be better spent by just making sure that you answer the question thoroughly.
In the electrolysis of molten sodium chloride, the reaction at the positive (anode) electrode would be:
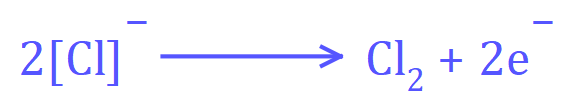
Negatively charged Chloride ions migrate towards the anode and when they get there they surrender an electron to form neutral Chlorine atoms. These neutral Chlorine atoms quickly power up covalently to form Chlorine gas which is released from the anode.
The reaction at the negative (cathode) electrode would be:
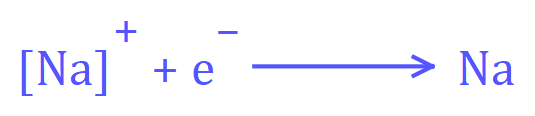
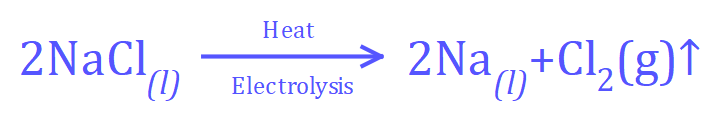
The overall reaction for the electrolysis of molten Sodium Chloride would be:
Go To >> Questions <<
Back To >> Electrolysis Of Molten Ionic Substances <<