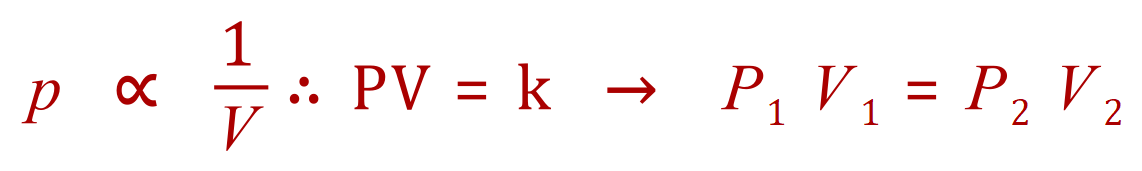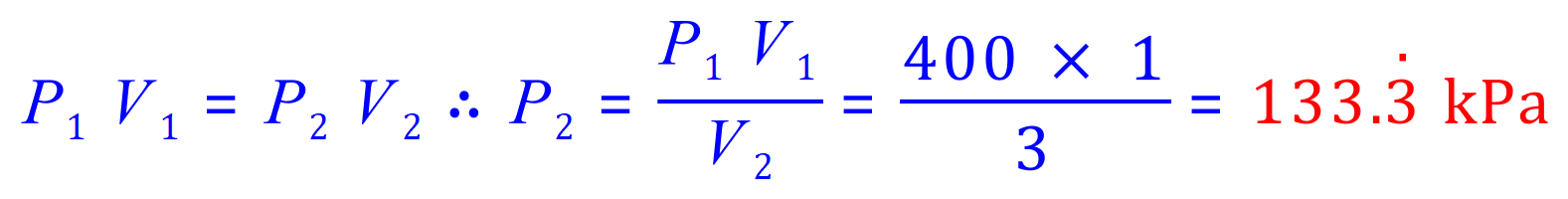Gas Laws
Note that the questions below have been sourced from many places, although it is less likely to be the case nowadays, it was previously quite normal for an intermix of old and new units, such as "atm" and "millimetres of mercury" (mmHg) for pressure, degrees Celsius and Kelvin for temperature. It is useful to know the relationship between old and new, so the information below (before the question star) will familiarise you.
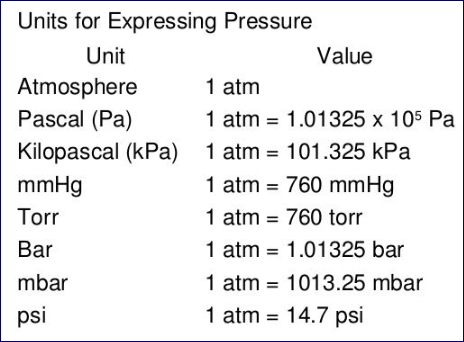
Pressure: 1 atm (atmosphere) = 101,325 Pa = 760 mmHg
Temperature: 0K = -273.15°C, 0°C = 273.15K
Volume: 1 L = 1000mL =1 dm3 = 1000 cm3
Boyle's Law
Q1. A fixed amount of a gas occupies a volume of 1L and exerts a pressure of 400 kPa on the walls of its container. What would be the pressure exerted by the gas if it is completely transferred into a new container having a volume of 3 litres (assuming the temperature and quantity of gas remains constant)?
It is a good idea to write out a small table, containing the 'known' values, then consider rearranging the appropriate equation in terms of the unknown (required) value:
P1=400kPa
P2= 'x'
V1=1L
V2=3L
Q2. A gas exerts a pressure of 3 kPa on the walls of container 1. When container 1 is emptied into a 10-litre container, the pressure exerted by the gas increases to 6 kPa. Find the volume of container 1. Assume that the temperature and quantity of the gas remain constant.
P1=3 kPa
P2=6kPa
V1='x'
V2=10L
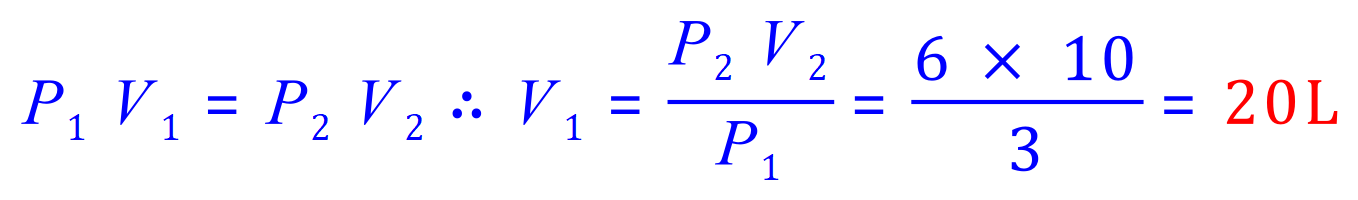
Q3. A balloon with a volume of 2.0 L is filled with a gas at 3 atmospheres. If the pressure is reduced to 0.5 atmospheres without a change in temperature, what would be the volume of the balloon?
P1= 3 atm
P2= 0.5 atm
V1= 2.0L
V2= 'x'
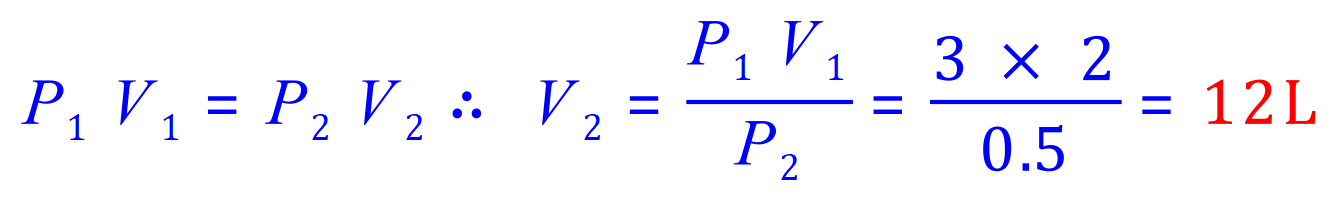
Charles' Law
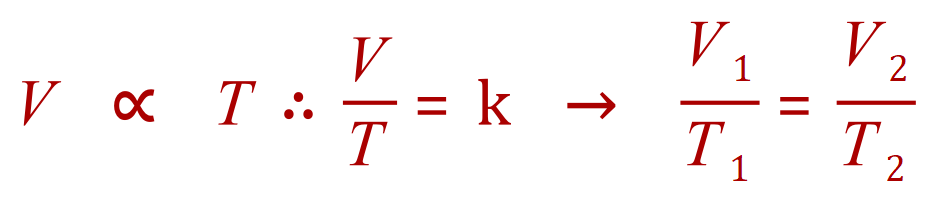
Q4. A 600 mL sample of nitrogen is heated from 27 °C to 77 °C at constant pressure. What is the final volume?
V1= 600mL
V2= 'x'
T1= (273+27) K - watch these, need to convert to K if given in C
T2= (273+77) K - watch these, need to convert to K if given in C
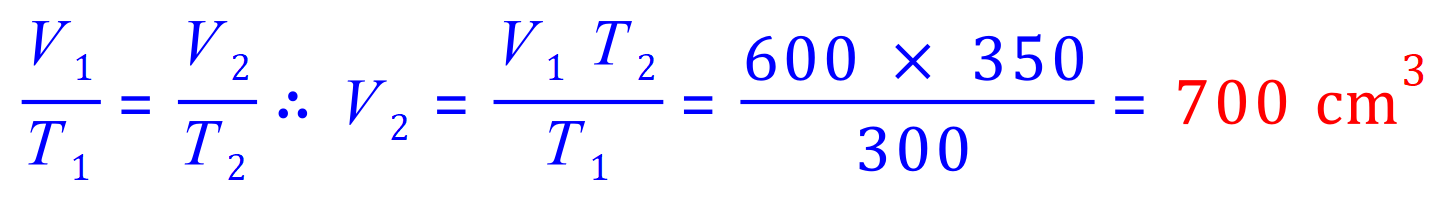
Q5. A gas occupies a volume of 400cm3 at 0°C and 780 mm Hg. What volume (in litres) will it occupy at 80°C and 780 mm Hg?
V1= 400 cm3
V2= 'x'
T1= (273+0) K
T2= (273+80) K
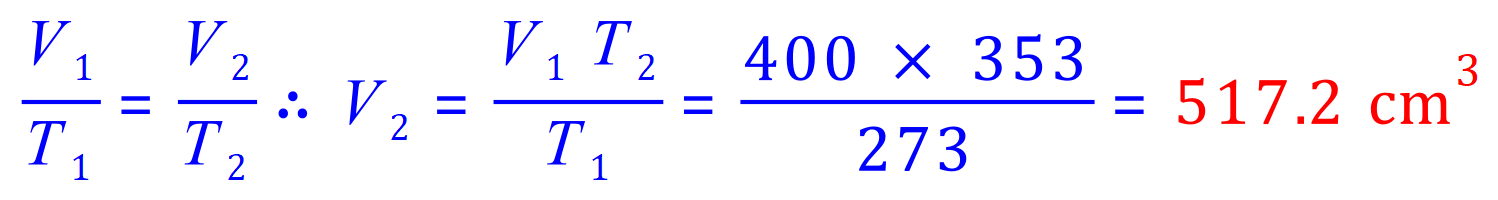
It's worth noting in this question 2 things, first of all you're given a pressure which doesn't vary which is therefore a little bit of a red herring because pressure is kept constant in Charles' law calculations. Secondly, you are given millimetres of mercury as opposed to atm or Pascal, it doesn't really matter provided you are consistent, if you wanted to you could multiply 780 x 101,325 and then divide the result by 760 to get the given pressure in Pascal, but there's no point as you don't need to use it anyway. The only thing you do need to be careful of in questions involving gases is the temperature, which if it is given in degrees Celsius MUST be converted to Kelvin before use.
Q6. Find the initial volume of a gas at 150 K, if the final volume is 6 L at 100 K
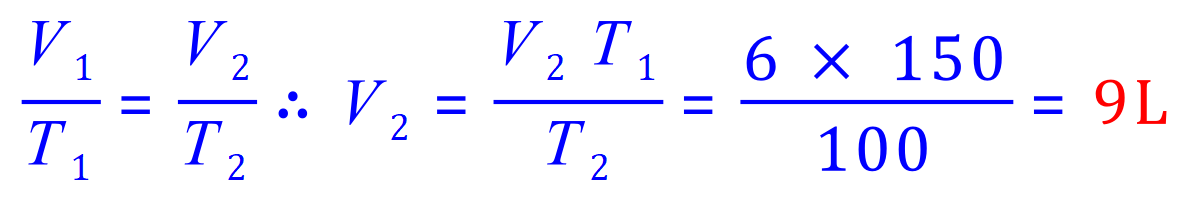
Gay-Lussac's Law
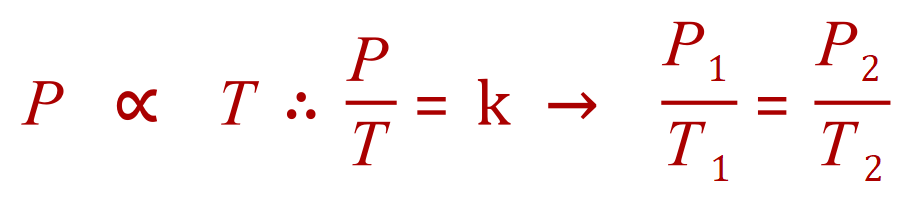
Q7. The pressure of a gas in a cylinder when it is heated to a temperature of 250K is 1.5 atm. What was the initial temperature of the gas if its initial pressure was 1 atm.
P1= 1 atm
P2= 1.5 atm
T1= 'x'
T2= 250K
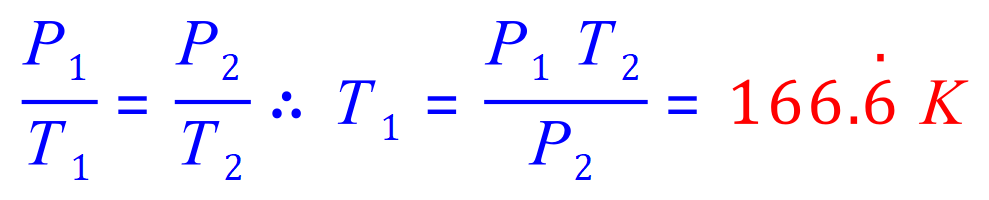
Once again, you are given "old" units, "atm" instead of "Pascal". There is no need to convert these as this will make no difference to the mathematics. Again, the only place where you need to be careful is when dealing with temperatures.
Q8. At a temperature of 300 K, the pressure of the gas in a deodorant can is 3 atm. Calculate the pressure of the gas when it is heated to 900 K.
P1= 3 atm
P2= 'x'
T1= 300 K
T2= 900 K
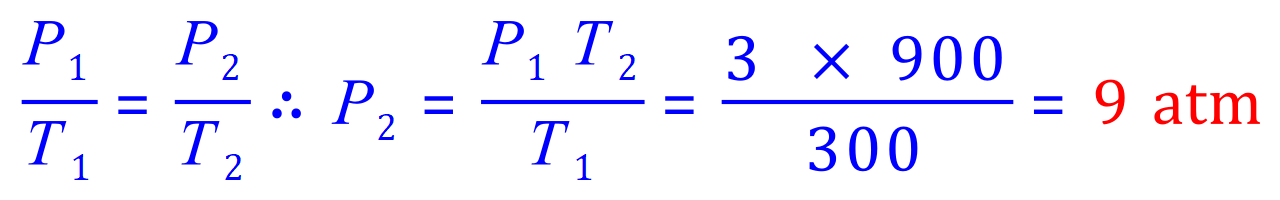
Once again, you are given "old" units, "atm" instead of "Pascal". There is no need to convert these as this will make no difference to the mathematics. Again, the only place where you need to be careful is when dealing with temperatures.
General Gas Equation
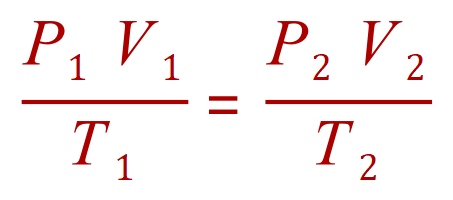
Q9. A gas is kept at a constant pressure while its temperature is varied. If it is initially at 20C and has a volume of 1 L exactly, calculate its volume at 60C.
(This is in fact a Charles' law question as the pressure P is constant)
V1= 1 L
V2= 'x'
T1= (273+20) K
T2= (273+60) K
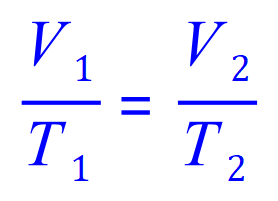
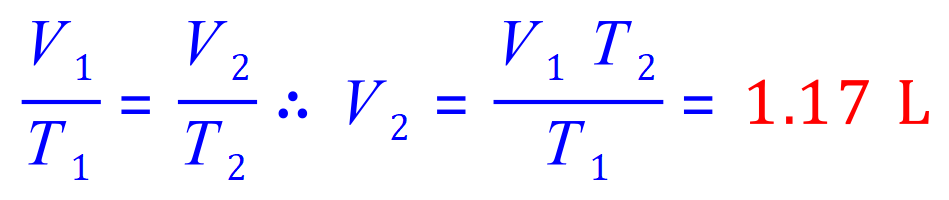
Note that the question does not state what units to use in your answer, in this case stay with the units given in your 'known' amounts, eg: L
Q10. A cylinder with a movable piston contains gas at a temperature of 27C, with a volume of 1.5 m3 and a pressure of 0.20 x 105 Pa. What will be the final temperature of the gas if it is compressed to 0.7 m3 and its pressure us increased to 0.80 x 105 Pa?
P1= 0.20 x 105 Pa
V1= 1.5 m3
T1= (273+27) K
P2= 0.80 x 105 Pa
V2= 0.7 m3
T2= 'x'
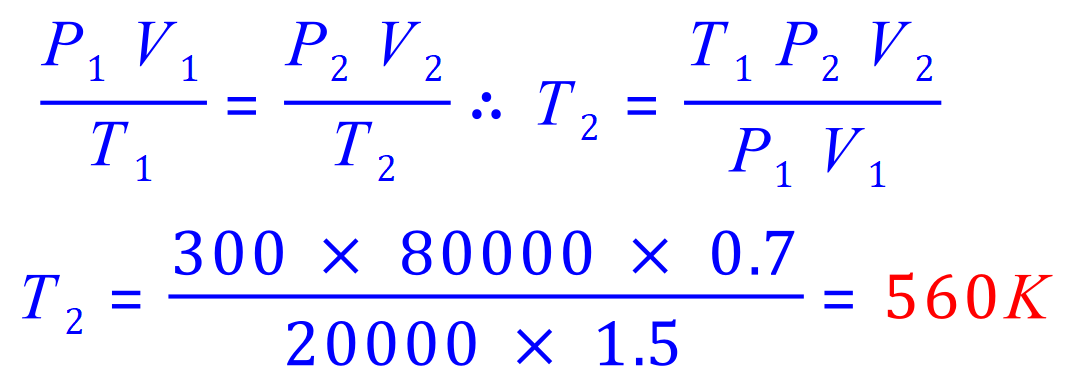
In this calculation I converted the exponents to integer values just to make the computation easier. Entering 20000 and 80000 is far simpler on a calculator than 0.2 x 105 and 0.8 x 105 and far less prone to errors.
Back To >> Questions <<
Back To >> Gas Laws <<