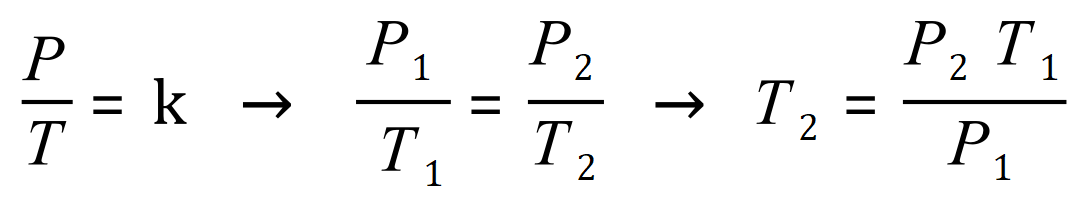Gay - Lussac's Law
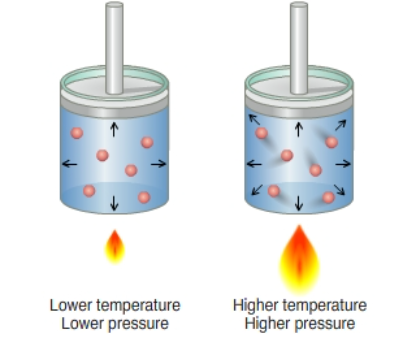
Gay Lussac's law (Amonton's law) states that if a quantity of gas is held at a constant volume, the pressure exerted by the gas will be proportional to the temperature applied. There will be a summary of these gas laws subsequently so that the nuances between them can be identified.
Let us take a look at this law as an equation:
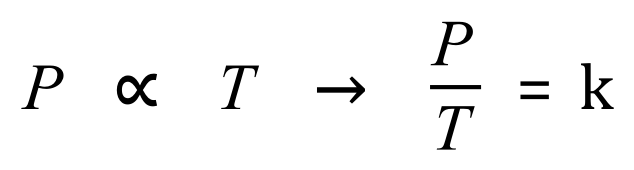
Q. A certain quantity of a gas is held in a rigid container with a constant volume, at 100kPa and a temperature of 450K. What will the temperature of the gas be if the pressure is increased to 350 kPa?
A. Remember to convert any given units if they are not in the expected format, we work in Pascal here so the pressures must be converted to Pa from kPa:
Given: