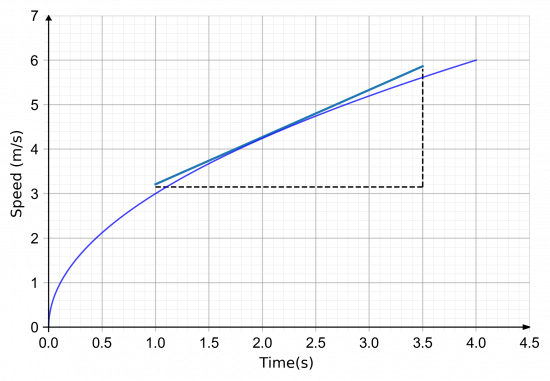
Instantaneous Acceleration
In chemistry, when we are looking at the rate of a reaction, such as the rate at which magnesium metal reacts with a dilute acid to give off hydrogen gas, we see that at the start of the reaction, gas is given off quite readily but as the reaction proceeds the rate appears to slow down until it stops. We can show this arithmetically by taking "instantaneous" readings of the rate of reaction at points in time, by drawing a tangent to the curve and calculating its gradient. We do exactly the same in a speed time graph to calculate the instantaneous acceleration at a given point.
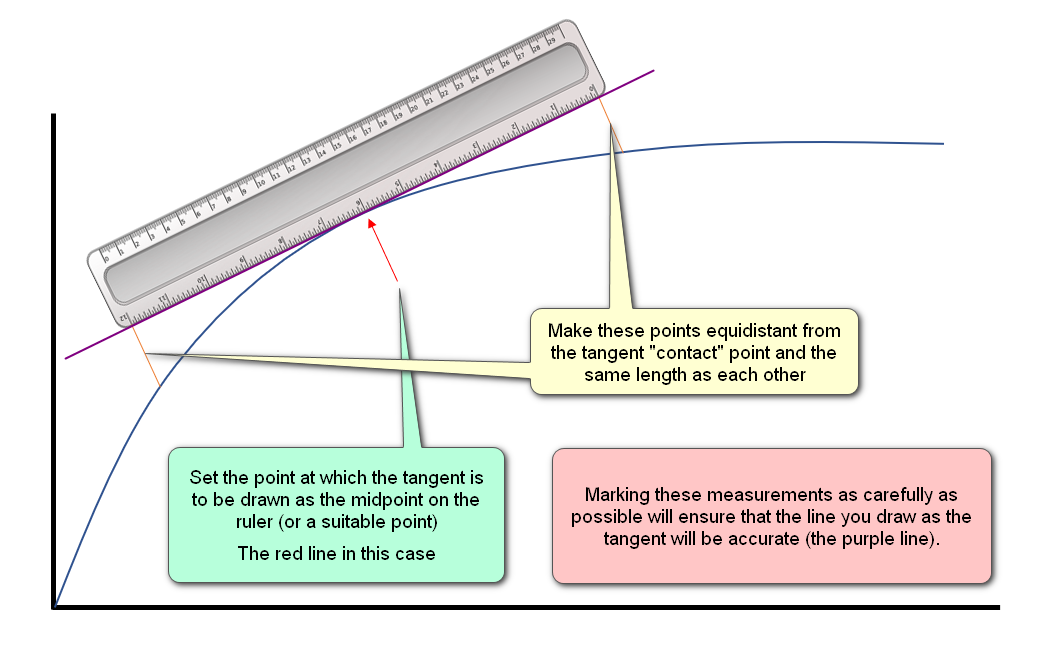
Great care must be taken when drawing these tangents, because if they are not drawn carefully and accurately the gradient calculation will be incorrect: