Radioactivity
You will need a periodic table or other access to Atomic Numbers to do these questions. You will be given the Atomic Mass but not the Atomic Number.
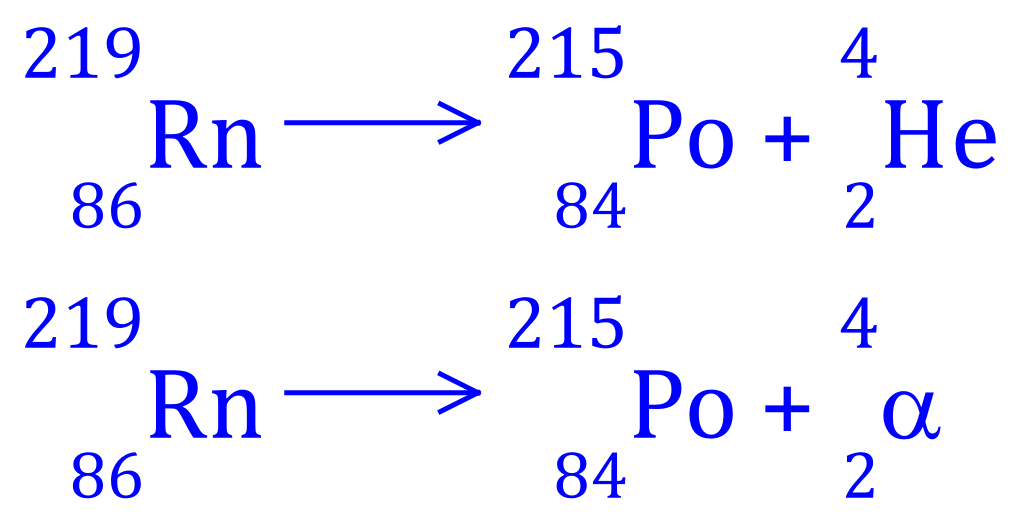
Q1. Radon-219 decays into Polonium 215, write the balanced equation for this nuclear process and state what type of radioactive decay is involved.
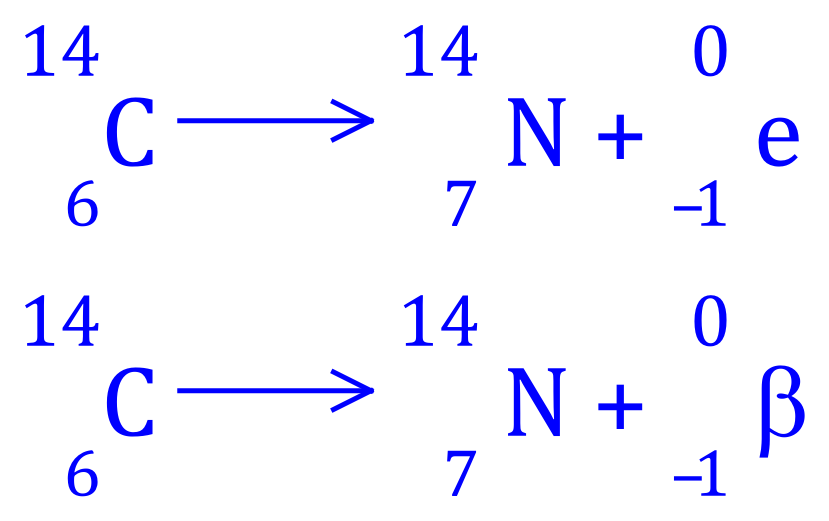
Q2. Carbon-14 decays to Nitrogen-14, write the balanced equation for this nuclear process and state what type of radioactive decay is involved.
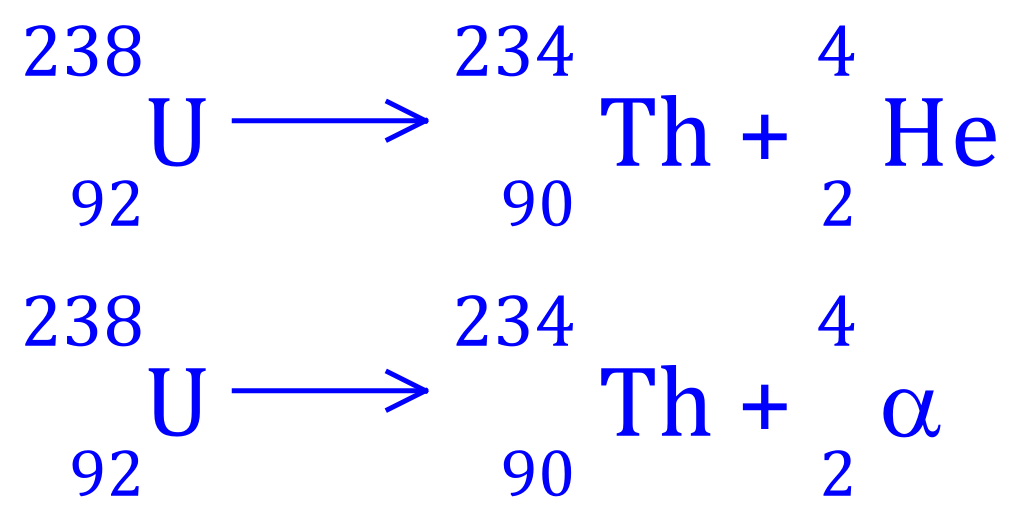
Q3. Uranium-238 decays to Thorium-234, write the balanced equation for this nuclear process and state what type of radioactive decay is involved.
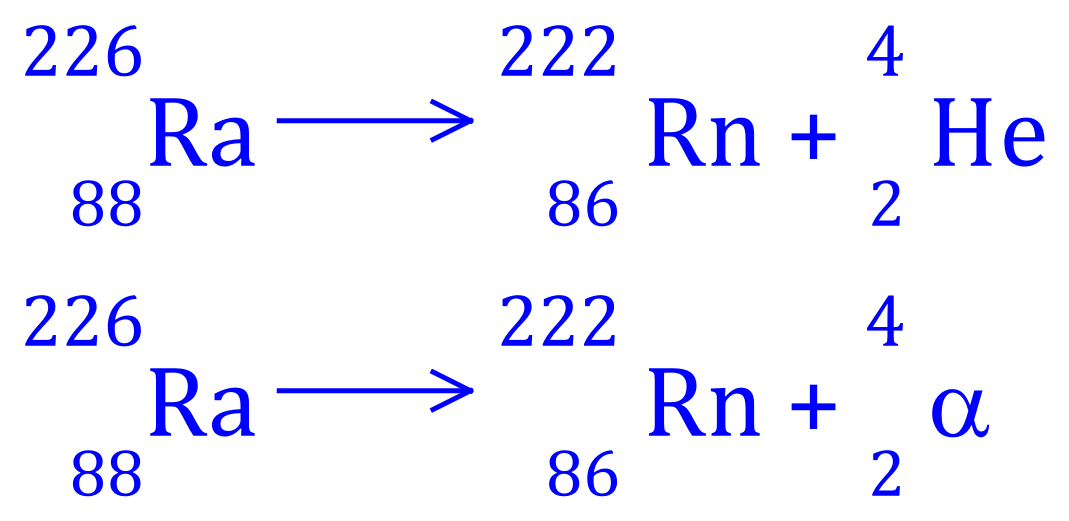
Q4. Radium-226 decays to Radon-222, write the balanced equation for this nuclear process and state what type of radioactive decay is involved.
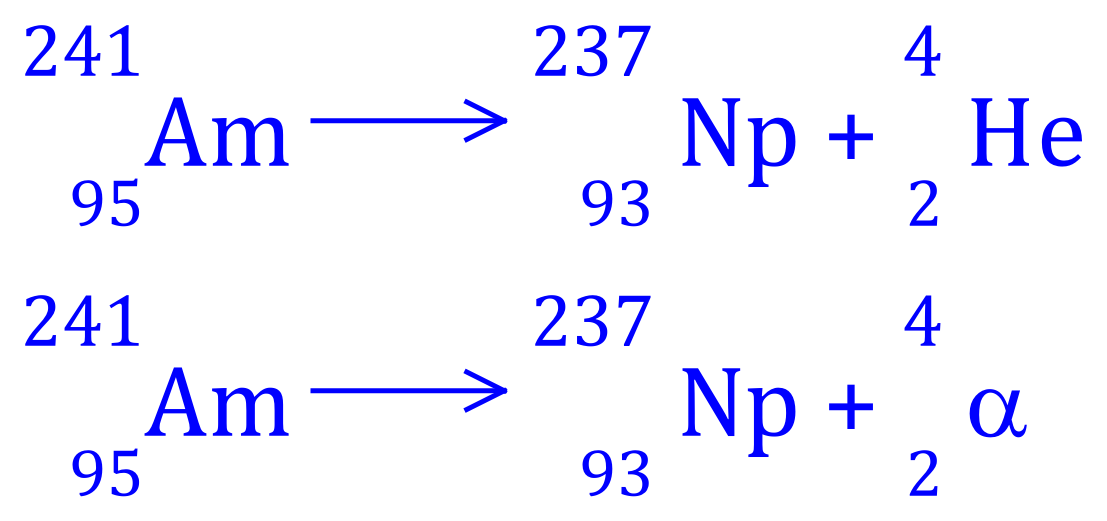
Q5. Americium-241 decays to Neptunium-237, write the balanced equation for this nuclear process and state what type of radioactive decay is involved.
Q6. Iodine-131 decays to Xenon 131, write the balanced equation for this nuclear process and state what type of radioactive decay is involved.
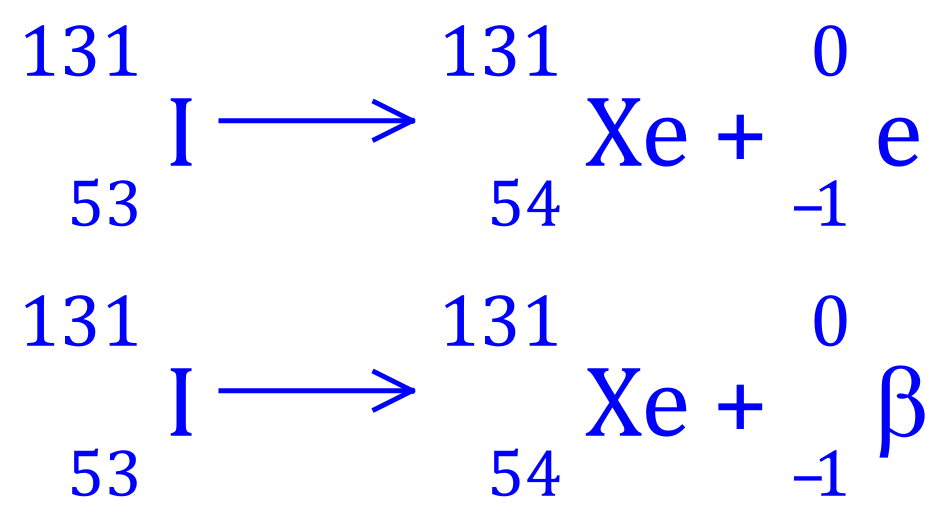
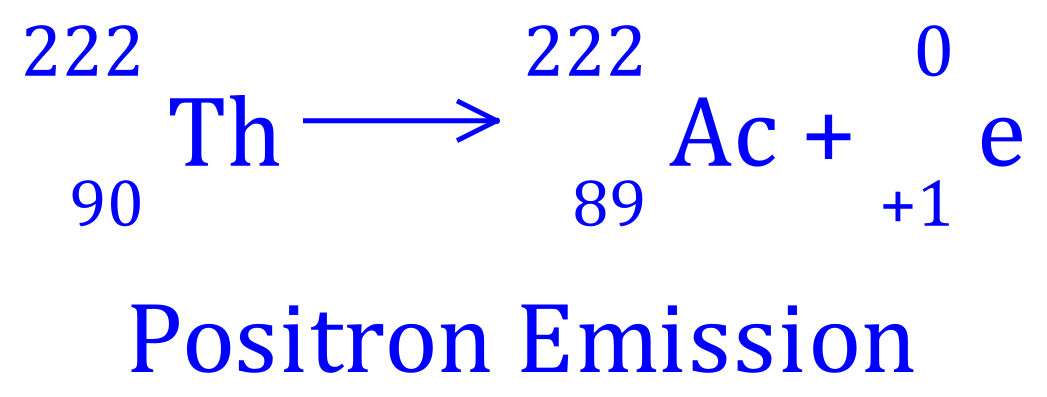
Q7. Thorium-232 decays to Actinium-231, write the balanced equation for this nuclear process and state what type of radioactive decay is involved.
Q8. Manganese-49 decays to Chromium-49, write the balanced equation for this nuclear process and state what type of radioactive decay is involved.

Back To >> Questions <<
Back To >> Radioactivity <<