Test for Protein
When we test for proteins we use the Biuret test (pronounced "Buy - You - Ret"). The food sample is prepared in the usual way, by crushing a small food sample using a pestle and mortar and then transferring the grounds to a beaker, we then add distilled water to dissolve some of the food. The solution is filtered so that the undissolved remains are trapped and we conduct our test on the filtrate.
We transfer small amount (approximately 2 cm³) of the substance to a test tube and add about the same amount of biuret solution. If the first sample contains protein the solution will change from its normal blue colour to a pink/purple colour. If there is no protein present the solution will remain blue.
This next part is "nice to know" not "need to know" at this level.
What the GCSE books don't tell you is exactly how this test works, and much of the chemistry is beyond the scope of GCSE but still worth knowing because it is an interesting subject in its own right.
The Biuret solution is made up of Sodium Hydroxide, Potassium Sodium Tartrate and Copper Sulphate solutions. In aqueous solution, Cu (II) ions have a distinctly blue coloration, you have probably already seen dilute solutions of copper sulphate which are a lovely blue colour. Down at the molecular level each Cu (II) ion is surrounded by six water molecules to form a complex known as "Hexa Aqua - Copper (II)" (left image).
|
|
|
The dotted line represents a dative, or coordinate bond with the bonding electrons are both provided by the oxygen atom (in pink) towards the copper atom (in gold).
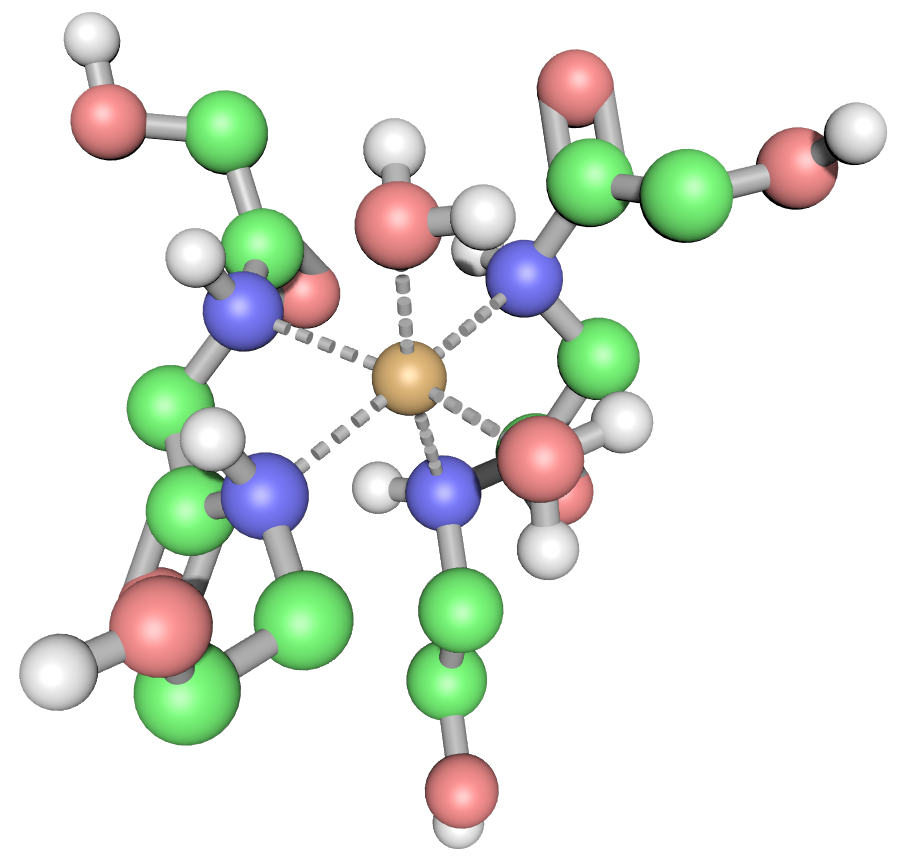
When we consider the positive result for the Biuret test, the traditional blue colour of the Hexa-Aqua Copper (II) ion as shown in the left image changes to a purple-mauve colour as a result of peptide bonds present datively bonding with the Cu2+ ion (via the lone pair of electrons on each nitrogen atom, shown in blue) displacing some of the Hex Aqua Copper water molecules (right-hand image).
|
|
Typical results of the Biuret test are pictured on the left. A positive test will provide a purple-mauve colouration due to the presence of the complex shown above right, whereas a negative result will retain the well-known blue colouration of the Hex Aqua Copper Ion (above left). |