Test for Starch
Another practical that you will probably do at some point is the test for Starch using Iodine solution. Prepare the food sample in the same way as before, mash your sample with a pestle and mortar and transfer the smashed up contents to some distilled water, mix it thoroughly and then filter off any undissolved solid that remains. Your tests will be performed on the filtrate.
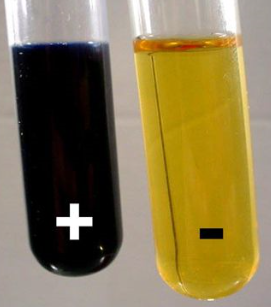
Iodine solution is usually made up using elemental Iodine dissolved in a solution of Potassium Iodide and is usually an orange brown colour. In the presence of starch the colour will change to blue black.
This next part is "nice to know" not "need to know" at this level.
What the GCSE books don't tell you is exactly how this test works, and much of the chemistry is beyond the scope of GCSE but still worth knowing because it is an interesting subject in its own right.
Dissolving elemental iodine in a solution of potassium iodide will produce the single negatively charged "triiodide" ion and "pentaiodide" ion..
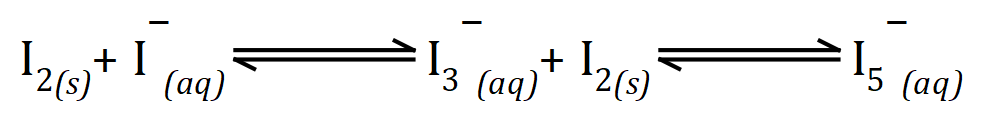
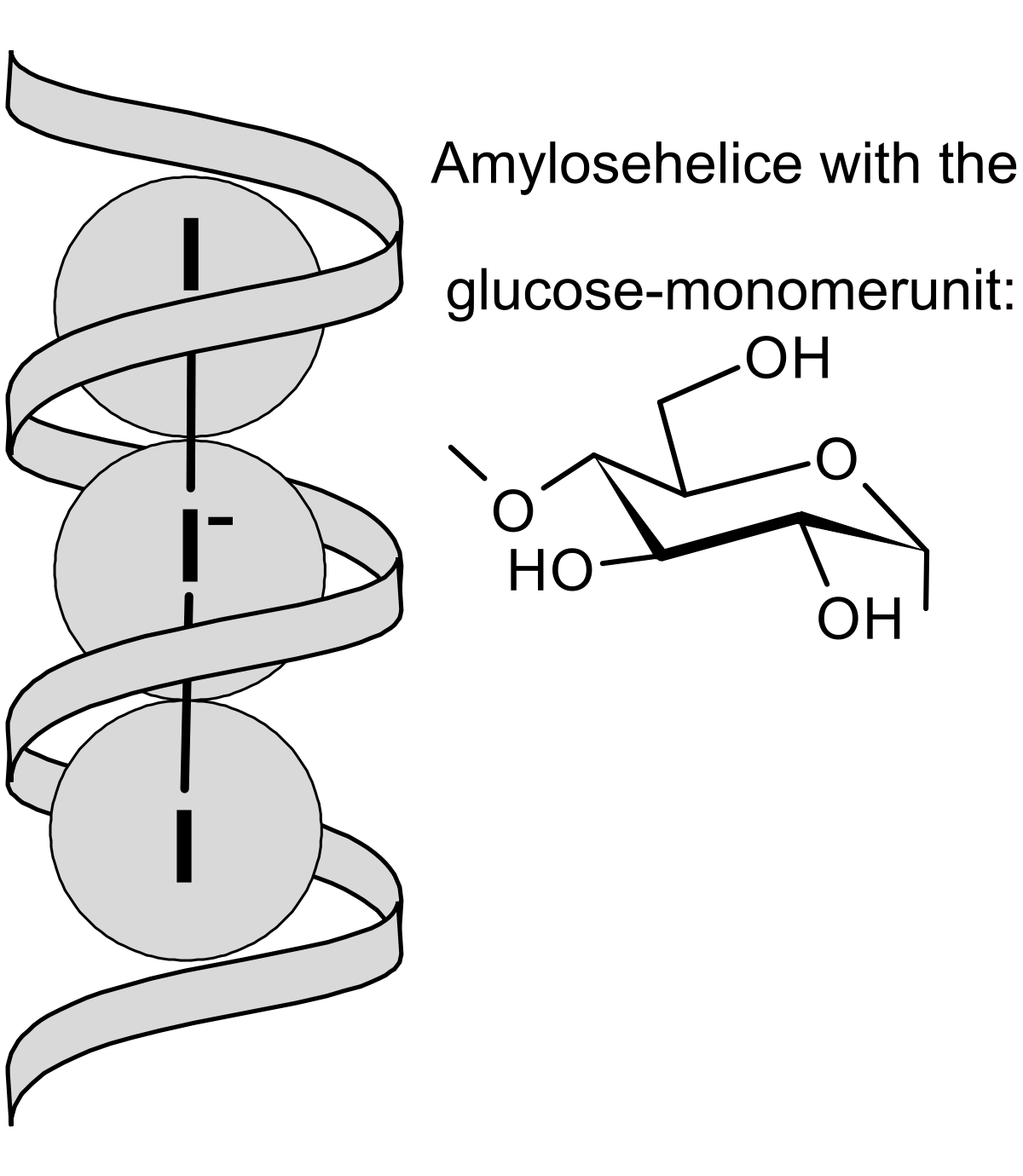
In the presence of starch (10-25% amylose and 75-90% amylopectin) the triiodide forms a dark almost black complex with the amylose which is normally found as a helix shaped molecule (think of DNA). The triiodide (and sometimes pentaiodide) ions are linear and lie inside the helical structure of the amylose.
|
|
|