Test for Sugars
Testing for sugars involves the use of a substance called Benedict's solution, and unsurprisingly also known as the Benedict test. Sugars are found in all sorts of food and there are two types, we have "reducing" sugars and "non-reducing" sugars. The Benedict test allows you to test for reducing sugars.
Prepare your food sample in the usual way and add approximately 5 cm³ of it to a clean test tube. Add approximately 10 drops of Benedict's solution and then place the test tube into a water bath at 75°C for about five minutes.
Benedict solution is made up of four substances:
1. Aqueous copper sulphate - this produces the copper (II) ions
2. Aqueous sodium carbonate - this makes the solution alkaline
3. Aqueous sodium citrate - this complexes with the copper (II) ions to prevent them degrading (being reduced) to copper (I) during storage
4. Distilled water - this is simply the solvent
Benedict solution is blue in colour (presumably due to the copper sulphate present in solution).
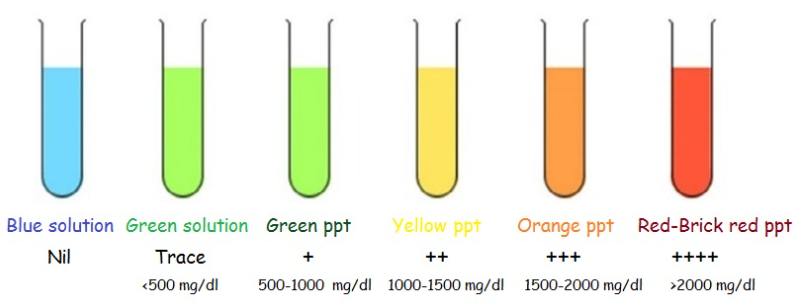
A typical result for the Benedict test is shown in the picture above with approximate amounts of reducing sugars present. The reason for the brick red coloration in the higher concentrations is due to the formation of Copper (I) Oxide by reduction of Copper (II) ions to Copper (I) ions:
This next bit is "nice to know" as opposed to "need-to-know" at this level.
Reducing sugars under alkaline condition tautomerise and form enediols. Enediols are powerful reducing agents. They can reduce cupric ions (Cu2+) to cuprous form (Cu+), which is responsible for the change in color of the reaction mixture. This is the basis of Benedict’s test. When the conditions are carefully controlled, the colouration developed and the amount of precipitate formed (Cuprous oxide) depends upon the amount of reducing sugars present.
(https://laboratoryinfo.com/benedicts-test-principle-reagent-preparation-procedure-interpretation - accessed 30.10.2019)