The Black Snake - Sugar and Sulphuric Acid
A fascinating experiment showing the way in which concentrated sulphuric acid can dehydrate sugar is this one. This is an experiment that MUST be conducted in a fume cupboard, it is very highly exothermic as the sugar is completely destroyed by dehydration leaving only very hot Carbon behind. In addition, some quite unpleasant by products are formed.
Sulphuric acid is mixed with ordinary household cane sugar, the white cane sugar starts to discolour as soon as the acid comes in contact with it, but it is a few seconds later that the reaction really gets started.

The power of the dehydration is evident by the fact that steam is produced, coming out of the mixture as the reaction proceeds. Not all of the water is lost this way, some remains in the tarry solution that is left.
The reaction is thus:
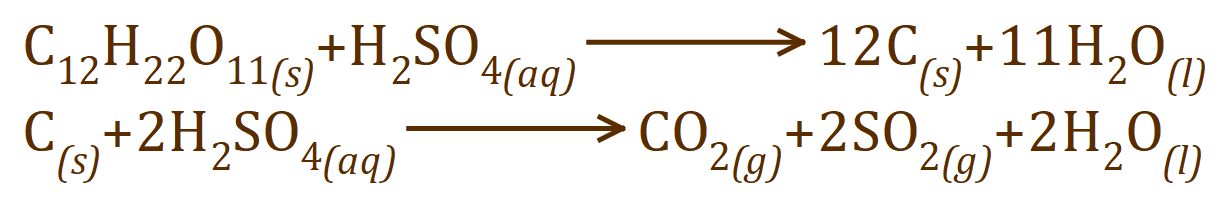
Although you would only expect Carbon and water, if there is an excess of sulphuric acid it can start to oxidise the Carbon to Carbon Dioxide, itself being reduced to oxides of Sulphur (Sulphur Dioxide as shown) and water.
The reason that this experiment is called the "Black Snake" should be evident, if you watch a video about this or the actual demonstration itself you will see the black snake arising from the beaker as the steam and gases produced push the elemental Carbon upwards.
This reaction has an enthalpy of reaction around the same magnitude as that of the "Screaming Jelly Baby" experiment.