The Chemical Volcano - Potassium (VII) Manganate and Glycerol
I can remember this experiment, as what could probably be regarded as the first "violent" chemical experiment I'd ever seen. Chemistry is full of weird and wonderful and in many cases extremely dangerous reactions and this one, being demonstrated by my chemistry teacher in the 1970s I found particularly fascinating.
Potassium (VII) Manganate, otherwise known by its old name of Potassium Permanganate reacts violently with Propan-1,2,3-triol , otherwise known as Glycerol or Glycerine according to the following, fairly complicated chemical equation:
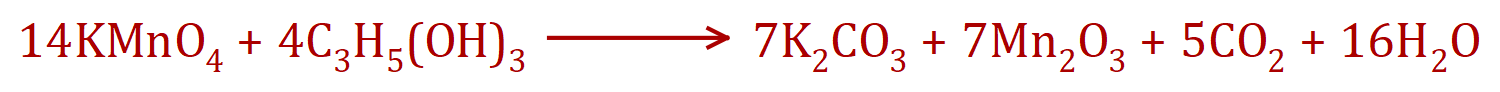
Crystalline potassium permanganate (KMnO4) is placed in an evaporating dish. A depression is made at the centre of the permanganate powder and glycerol liquid is added to it. The white smoke-like vapour produced by the reaction is a mixture of carbon dioxide gas and water vapour.
Note that this reaction can take a short time to get started, in any case it MUST be conducted in a fume cupboard.

Since the reaction is highly exothermic, initial sparking occurs, followed by a lilac- or pink-coloured flame.When energy or heat is added to electrons, their energy level increases to an excited state. This state is short-lived, and once the electrons release the energy, they return to their normal energy levels.
During this process the energy is visibly observed as light. When the reaction is complete, it leaves behind a greyish solid with green regions.