The Fountain Experiment - Solubility of Certain Gases in Water
A favourite experiment amongst secondary school science teachers (well, at least when I was at school in the 1970s it was quite popular) is the "Fountain Experiment" which was used to show the solubility in water of certain gases.
The diagram below is intended to be generic, but the favourite gas of choice appeared to be Ammonia. If we were to use, for example phenolphthalein in the bottom beaker, the colour of the solution would not be green as shown but it would be colourless, changing to pink under alkaline conditions. With this in mind, let's take a look at what happens to produce the effect.
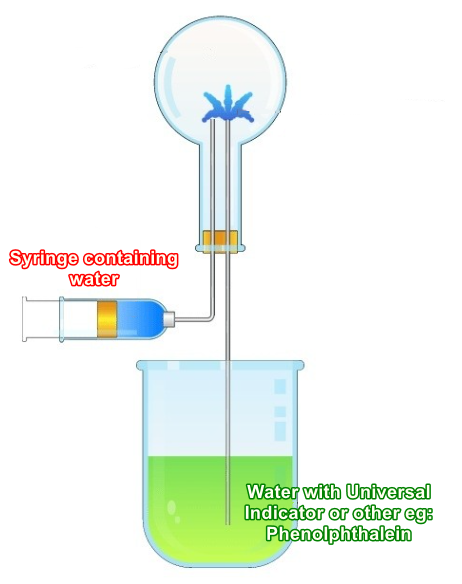
A mixture of water and phenolphthalein indicator (or universal indicator as shown in the diagram) is placed in the beaker as shown, with the rest of the apparatus as shown. The upper inverted round bottom flask is filled with ammonia gas and sealed with a rubber bung through which two glass tubes are inserted as shown.
Ammonia is very soluble in water, pause at this point and think about what might happen if water was introduced into the round bottom flask by the syringe? Well, a small amount of water is introduced to the flask from the syringe, to "seed" the reaction. As soon as the ammonia is exposed to the small amount of water, it dissolves to form ammonium hydroxide solution which, being alkaline will turn the indicator/phenolphthalein contained in the water to the appropriate colour to depict alkaline conditions (in the case of phenolphthalein, it will turn pink).
The round bottom flask is sealed, but now that some of the gas has dissolved into the water, there will be a small partial vacuum produced through the dissolution of the ammonia gas. This small partial vacuum will start to draw more water, this time from the beaker, up the second glass tube and into the flask. The rapid dissolution of more ammonia into this water will cause a considerable vacuum to be produced which will in turn continue to draw water from the beaker. The outcome is a "fountain" as shown in the picture until all of the gas has been dissolved.
The equation for the reaction is this:
