The Elephant's Toothpaste
Hydrogen Peroxide is a corrosive, irritating substance usually found in weak solution in water and has the chemical formula H2O2. It decomposes slowly over time into water and oxygen gas, this is accelerated by sunlight which is one of the reasons why you will usually find it in dark coloured, vented-cap bottles:

Commercial Hydrogen Peroxide has a limited shelf life because of this however certain catalysts can cause the breakdown to occur far more rapidly, producing water and evolving lots of oxygen gas. As we can't see oxygen gas, it's hard to gauge the rate of the reaction so one way to do this is to add a little bit of soapy water (washing-up liquid, shampoo et cetera) and a little bit of food colourant just to make it look pretty. Once this is all mixed together with a suitable catalyst, huge quantities of coloured oxygenated foam will be produced hence the name of the experiment "elephant toothpaste".
10 to 35% H2O2 solution
Dish soap (washing up liquid, liquid soap etc)
Food colourant(s)
Yeast/Warm water.
A solution of Hydrogen Peroxide is mixed with food colourant and washing-up liquid/shampoo/bubble bath and mixed. A suitable catalyst can be powdered brewers yeast for example, mixed in very warm water until it is a thin, pasty consistency, or a solution of Potassium Iodide in water. When the solutions are mixed together, the reaction is rapid and remarkable!

So, why does this work so well?
Let's look at the chemical structure/bonding in a Hydrogen Peroxide molecule:
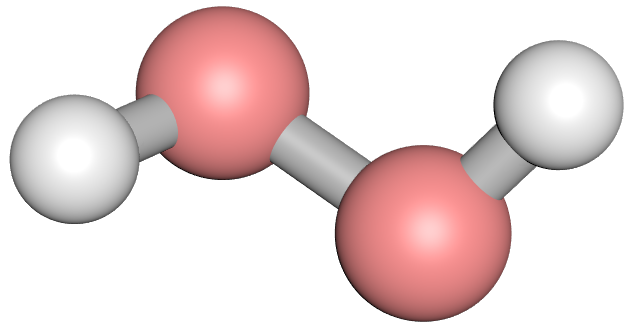
Normally oxygen molecules will bond to each other with double bonds, and this is a very stable arrangement, however occasionally oxygen to oxygen bonds will be single bonds known as "peroxide" bonds but these are very unstable and easy to break down. The breaking of the peroxide bond is an exothermic reaction.

It is the sheer speed at which this reaction takes place, when catalysed by something like Yeast or Potassium Iodide, and the visibility given to the gas produced by the soap solution which makes the "Elephants Toothpaste" such a fascinating reaction.